Solid Biosciences Wins FDA Orphan Drug Status for Friedreich's Ataxia Treatment
Solid Biosciences receives FDA Orphan Drug designation for SGT-212, a dual-route gene therapy treating Friedreich's ataxia, with first patient dosed in clinical trial.
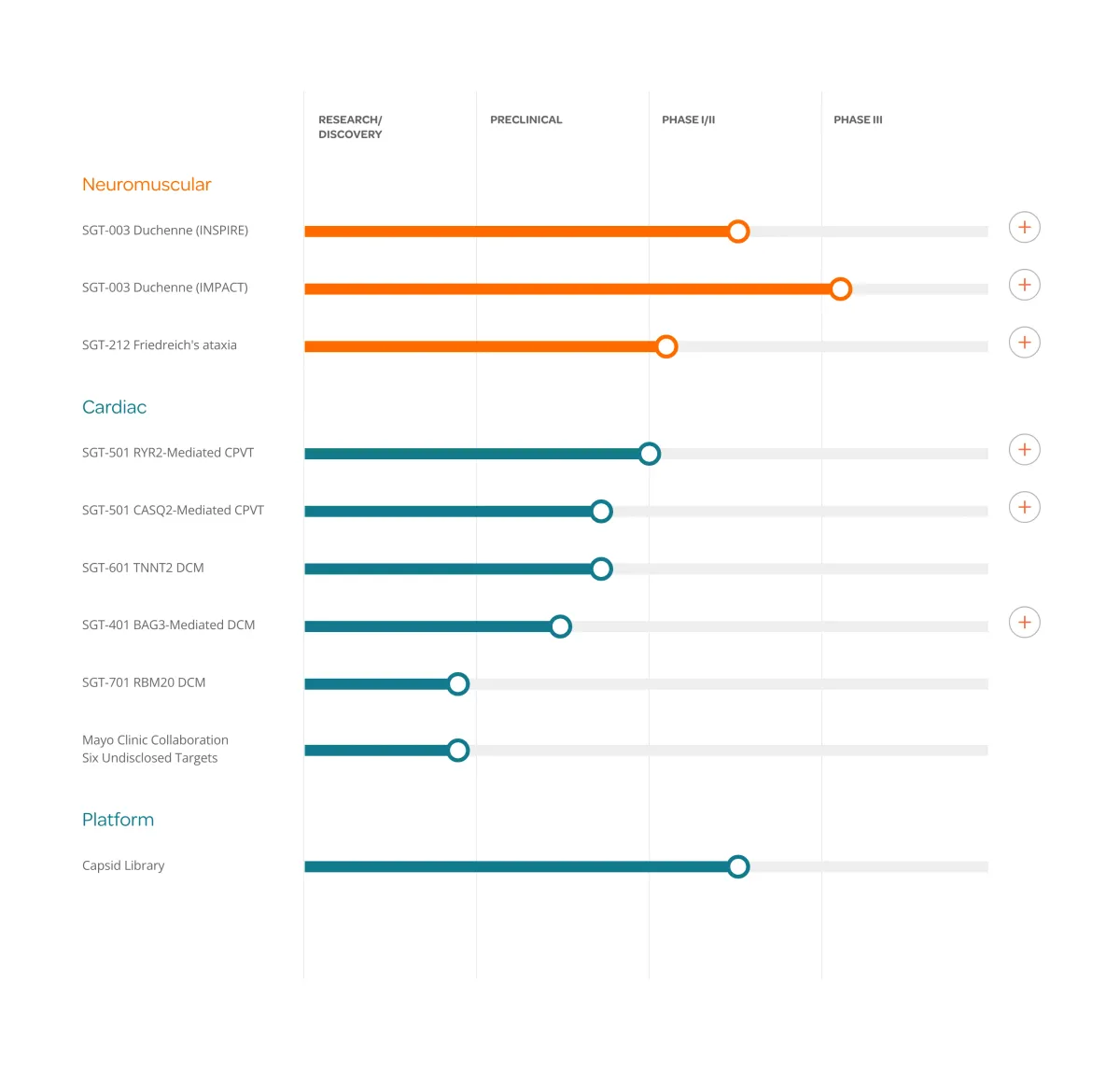
Solid Biosciences receives FDA Orphan Drug designation for SGT-212, a dual-route gene therapy treating Friedreich's ataxia, with first patient dosed in clinical trial.


Jefferies analyst highlights a key DOJ opinion supporting the use of the Defense Production Act to bypass California laws halting offshore oil production and pipelines for Sable Offshore.

Broadcom confirms major AI customers will stick with copper cables in racks through 2028, boosting Credo shares while Lumentum and Coherent dip after Nvidia's optics investments.

American Eagle Outfitters reports record Q4 sales up 10%, driven by Aerie's 23% comp growth and smart marketing. Shares dip despite beating estimates; FY2026 forecasts mid-single-digit sales rise amid tariff challenges.