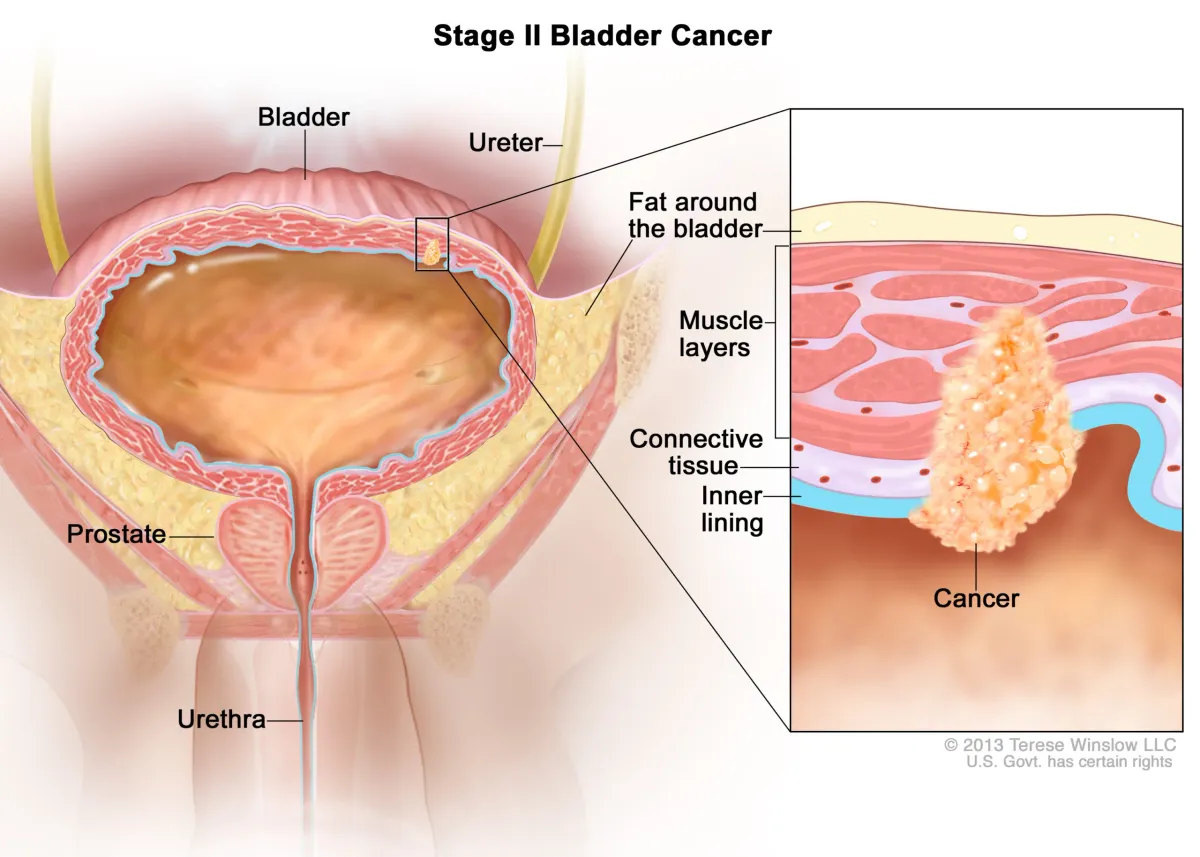
ImmunityBio Moves Forward with FDA on Bladder Cancer Treatment ANKTIVA
ImmunityBio advances FDA talks for ANKTIVA in papillary bladder cancer. Treatment shows 96% survival & 80% avoid bladder removal at 3 years, offering hope vs. current option: invasive surgery.
Already have an account? Sign in.



