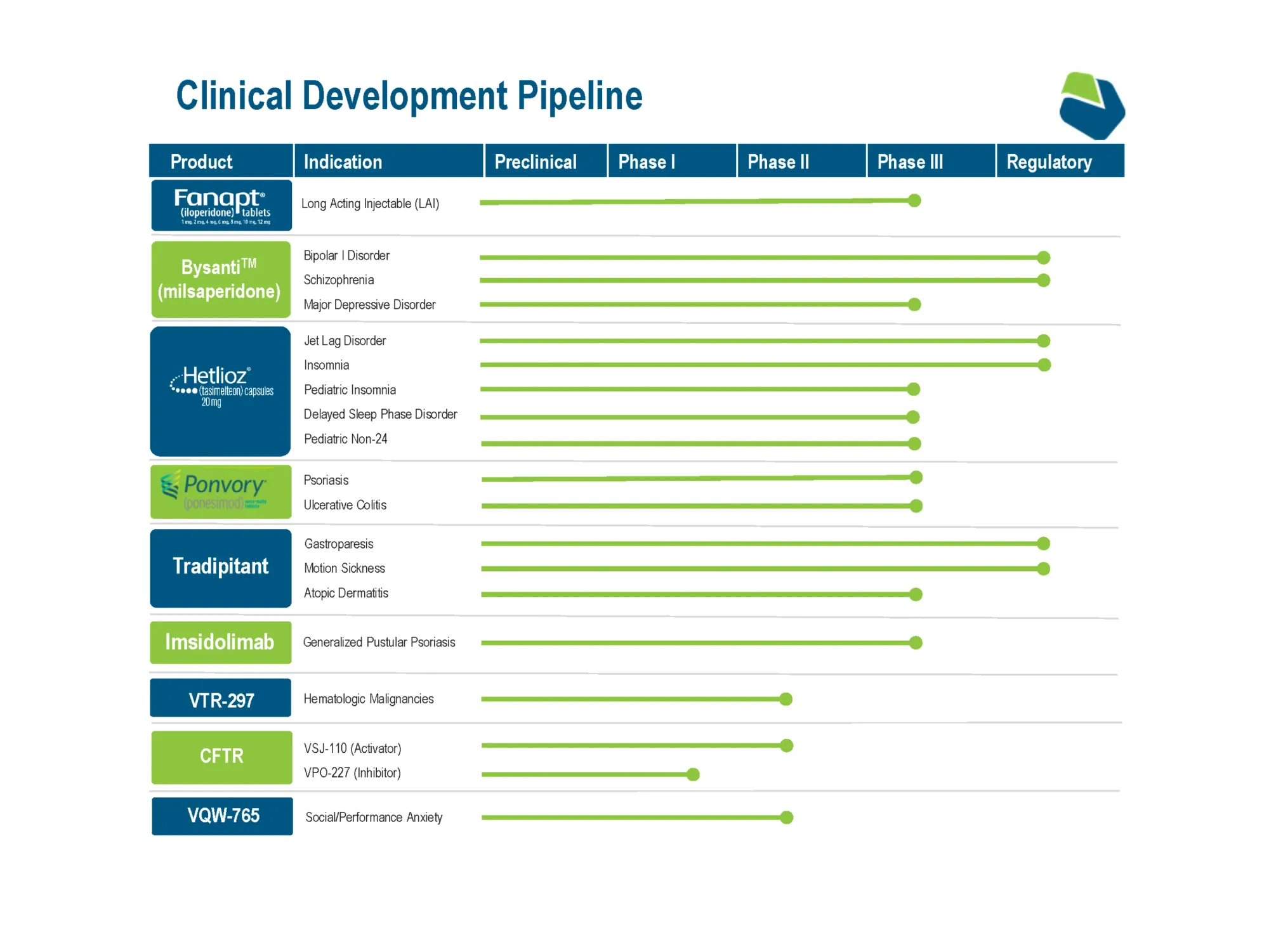
FDA Approves BYSANTI (Milsaperidone) for Bipolar I & Schizophrenia
Vanda Pharmaceuticals’ BYSANTI (milsaperidone) receives FDA approval as a new atypical antipsychotic for bipolar I manic/mixed episodes and schizophrenia in adults. Launch expected Q3 2026 with strong patent protection until 2044.