
FDA Extends Review Timeline for Travere's FSGS Treatment
FDA delays approval decision for Filspari in rare kidney disease FSGS until April 2026. Travere Therapeutics (TVTX) continues commercial preparations for potential first FSGS treatment.

FDA delays approval decision for Filspari in rare kidney disease FSGS until April 2026. Travere Therapeutics (TVTX) continues commercial preparations for potential first FSGS treatment.
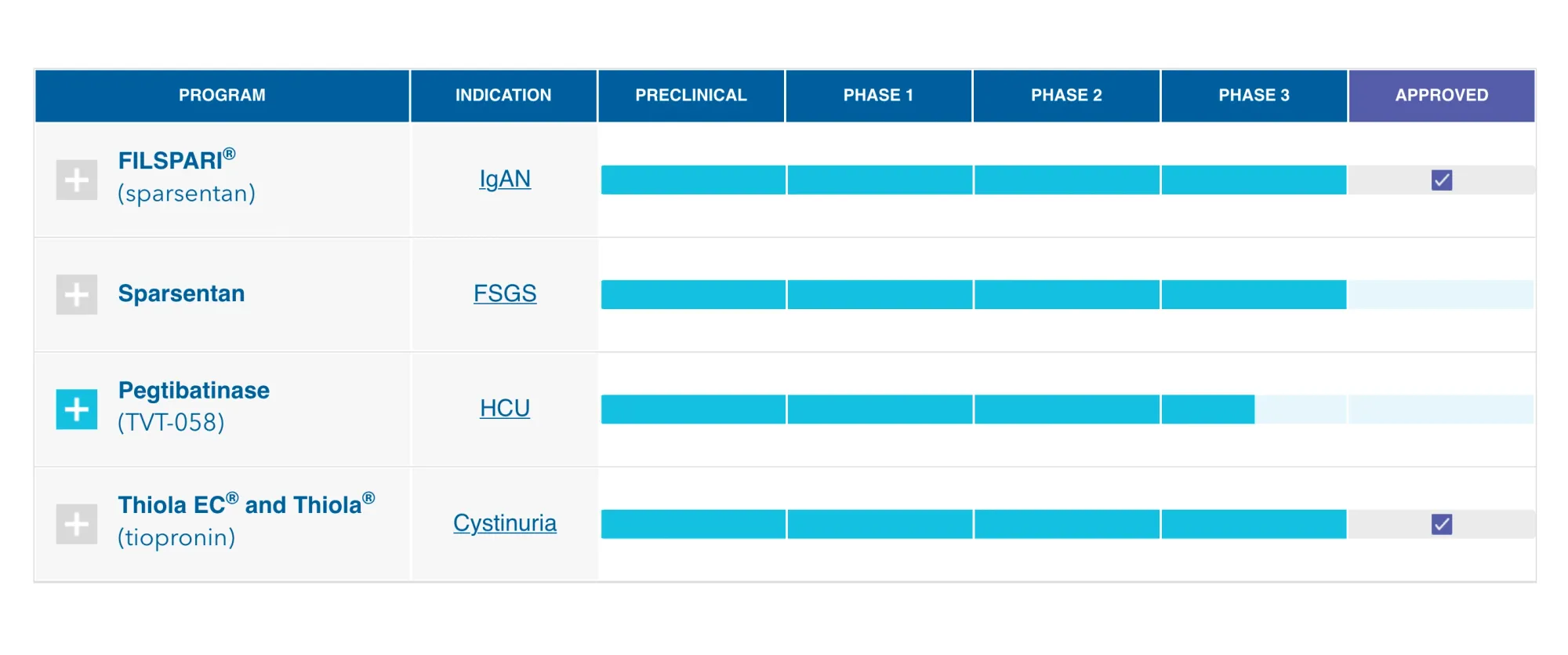
Travere Therapeutics shares drop 33% in premarket as FDA requests more data for FILSPARI FSGS approval, delaying potential first-ever treatment for rare kidney disease.

FDA confirms proteinuria reduction as valid FSGS approval endpoint in Dimerix feedback, boosting confidence in Travere Therapeutics' upcoming Filspari decision.

Travere Therapeutics shares jumped 15% after reporting a strong Q3 profit of $25.7M, driven by surging FILSPARI sales and better-than-expected earnings. Citi raised its price target to $48, citing optimism for FDA approval in early 2026.
FILSPARI (sparsentan) for rare kidney disease FSGS is close to FDA approval, with no advisory committee needed! It's the 1st potential treatment, showing strong trial results by reducing protein in urine and lowering kidney failure risk. PDUFA date: Jan 13, 2026.