
Sangamo Therapeutics Raises $25M Through New Stock Offering
Sangamo Therapeutics announces a $25 million stock and warrant offering to strengthen its finances and support general business needs.

Sangamo Therapeutics announces a $25 million stock and warrant offering to strengthen its finances and support general business needs.

Sangamo Therapeutics (SGMO) receives FDA Fast Track designation for ST-503, a promising non-opioid treatment for small fiber neuropathy causing chronic pain.

Sangamo Therapeutics receives FDA approval to submit rolling application for ST-920, a one-time gene therapy treating Fabry disease, with accelerated review pathway granted.
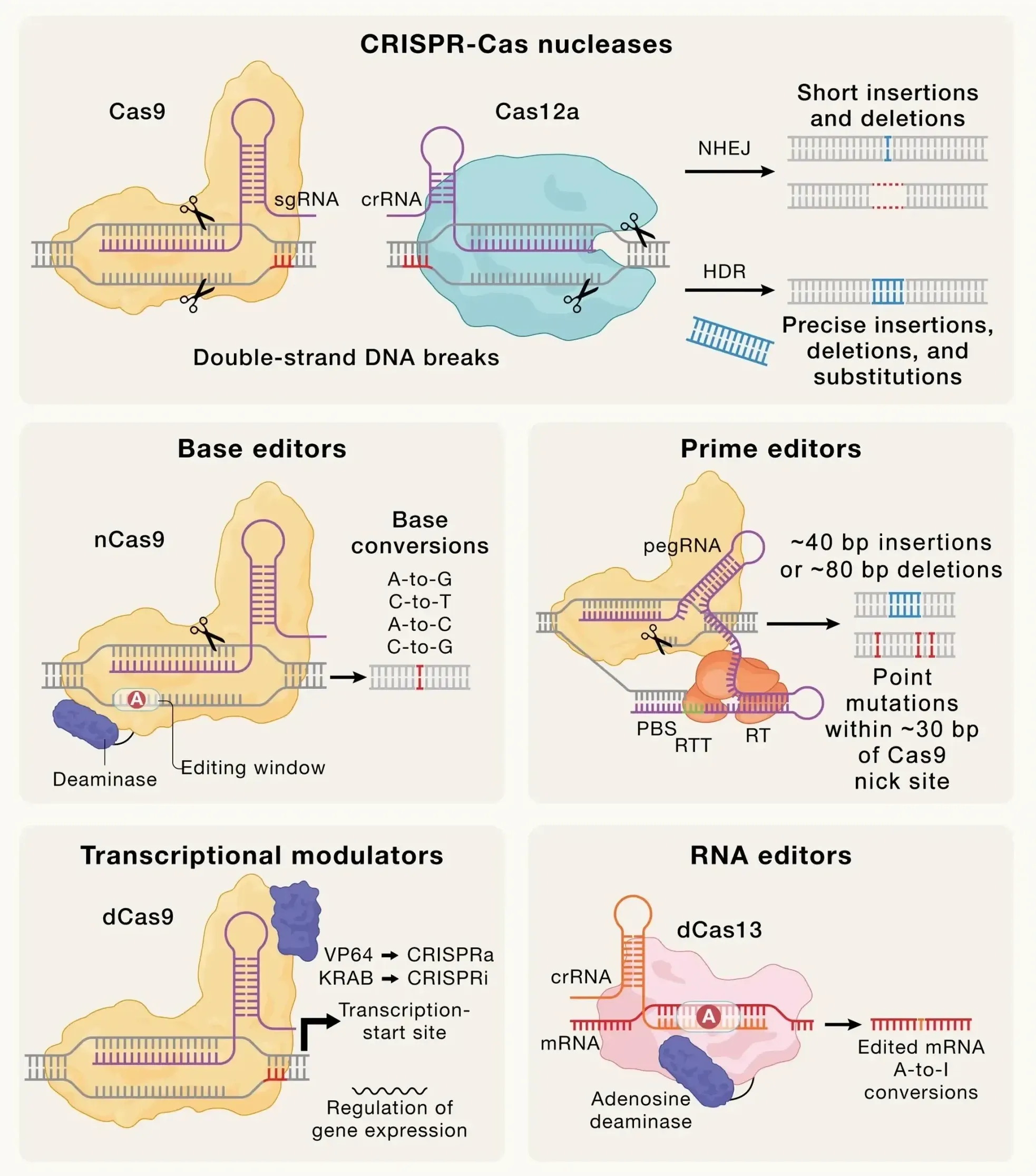
The FDA is unveiling a faster approval pathway for custom gene-editing therapies, aiming to spark major investment and accelerate cures for rare diseases through Crispr-based innovations.