GSK Acquires RAPT Therapeutics for $2.2 Billion to Develop New Food Allergy Treatment
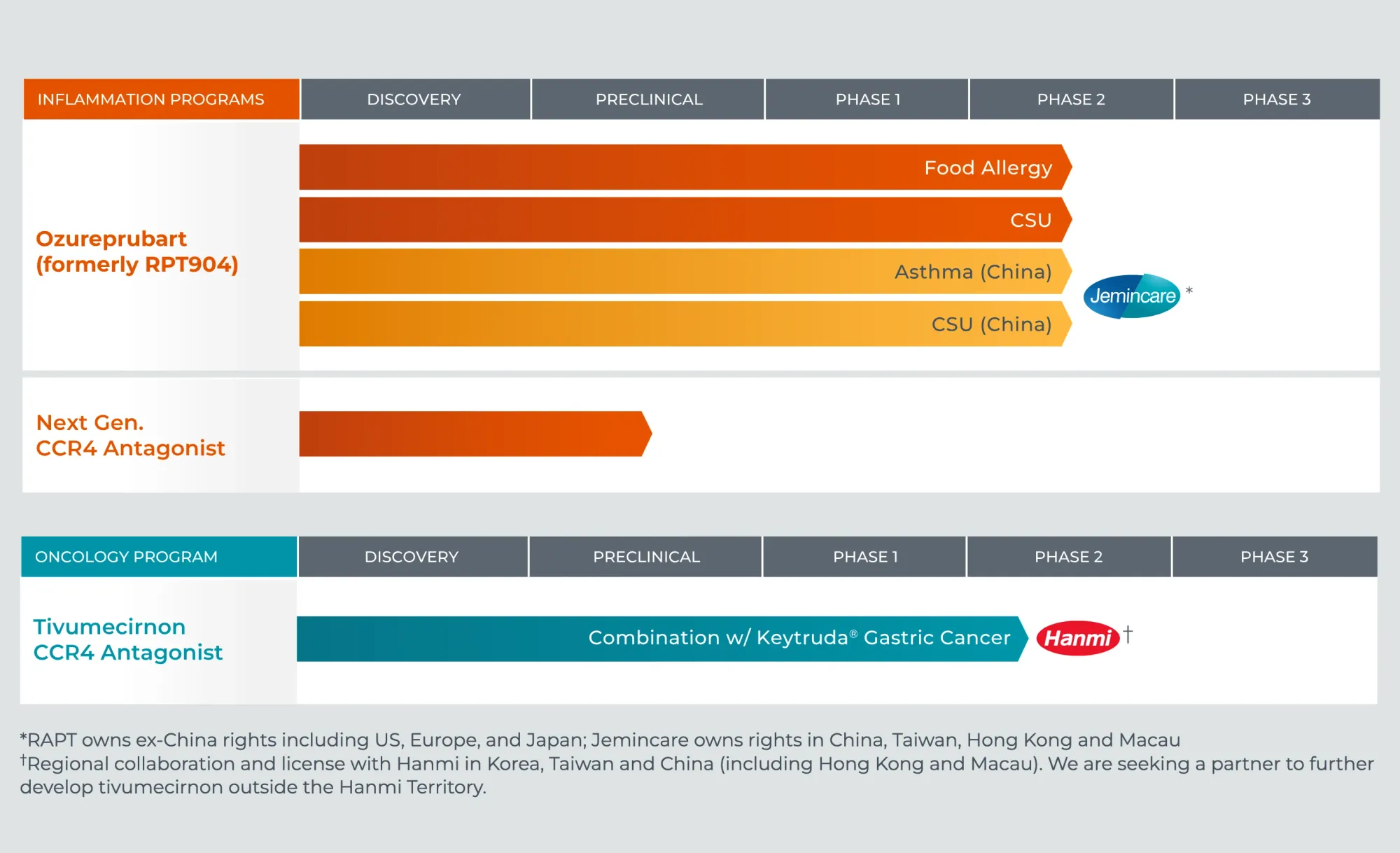
GSK purchases RAPT Therapeutics for $2.2B, gaining ozureprubart - a promising food allergy treatment requiring injections every 12 weeks instead of every 2 weeks.

GSK purchases RAPT Therapeutics for $2.2B, gaining ozureprubart - a promising food allergy treatment requiring injections every 12 weeks instead of every 2 weeks.

JPMorgan raises RAPT stock rating to Overweight with $55 target after Phase 2 trial success. Stock jumps 17% on positive chronic urticaria treatment data.

FDA OKs major trial (prestIgE) for RPT904, a next-gen anti-IgE drug. It will treat ~100 people allergic to peanut, milk, or egg. The goal is a better, safe therapeutic option by blocking IgE, the key driver of these allergies.

Leerink Partners dramatically upgraded Rapt Therapeutics stock based on the strong outlook for RPT904, a leading drug candidate designed to treat severe allergies, including food allergy, by improving on the current standard treatment, Xolair.