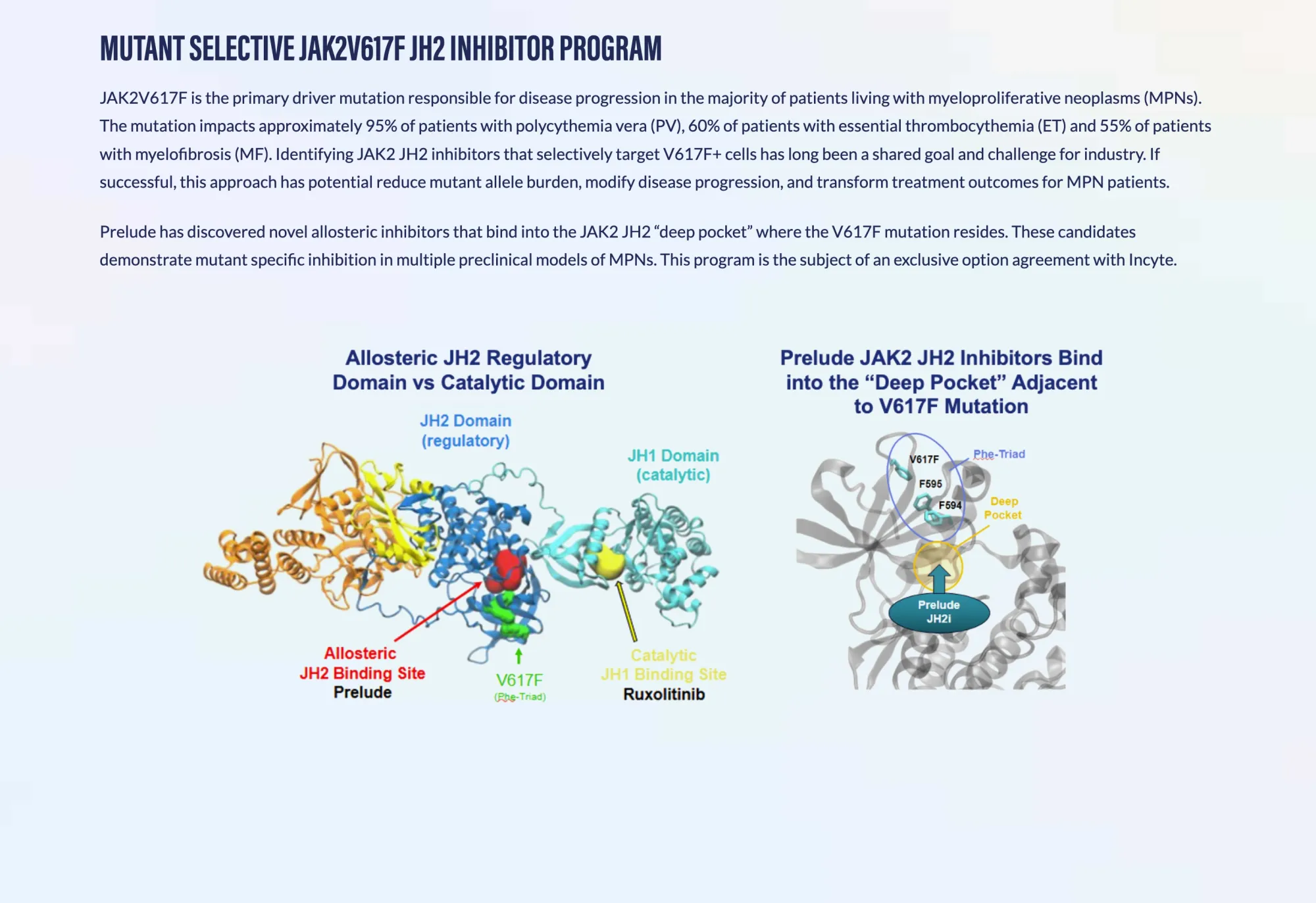
Prelude Therapeutics Gets FDA Green Light for Groundbreaking Blood Cancer Treatment
Prelude Therapeutics receives FDA clearance to begin testing PRT12396, a targeted therapy for myeloproliferative neoplasms affecting thousands of patients.

Prelude Therapeutics receives FDA clearance to begin testing PRT12396, a targeted therapy for myeloproliferative neoplasms affecting thousands of patients.
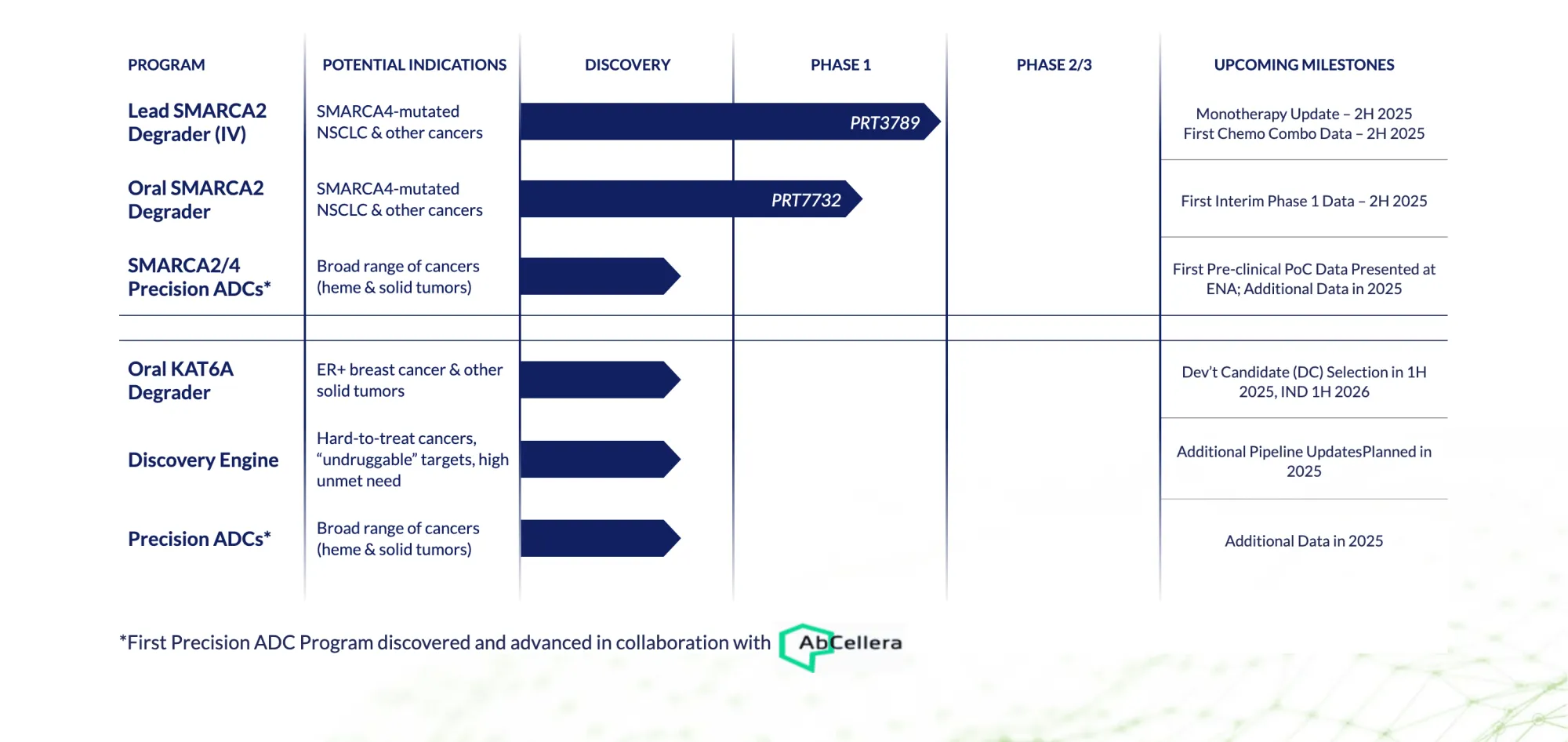
Prelude Therapeutics streamlines its oncology pipeline — prioritizing JAK2V617F and KAT6A programs, pausing SMARCA2, and securing major funding through an Incyte partnership.