Ulixacaltamide Receives FDA Breakthrough Designation for Tremor Treatment
Praxis Precision Medicines receives FDA Breakthrough Therapy Designation for ulixacaltamide, a promising new treatment for essential tremor affecting 7 million Americans.
Praxis Precision Medicines receives FDA Breakthrough Therapy Designation for ulixacaltamide, a promising new treatment for essential tremor affecting 7 million Americans.
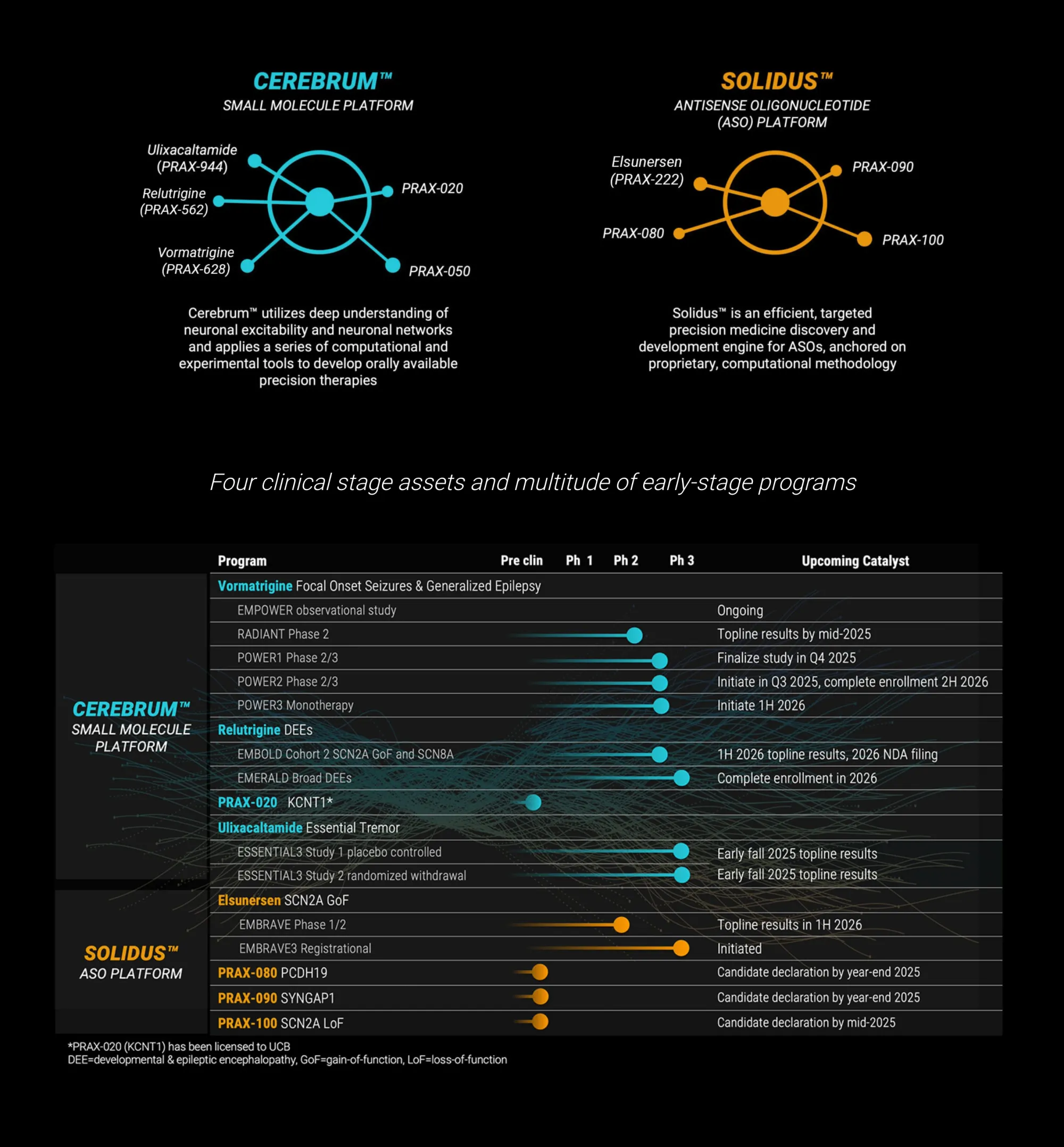
BTIG raises its price target on Praxis Precision to $843, citing strong prospects for its essential tremor drug Ulixacaltamide and major growth potential ahead of 2026.
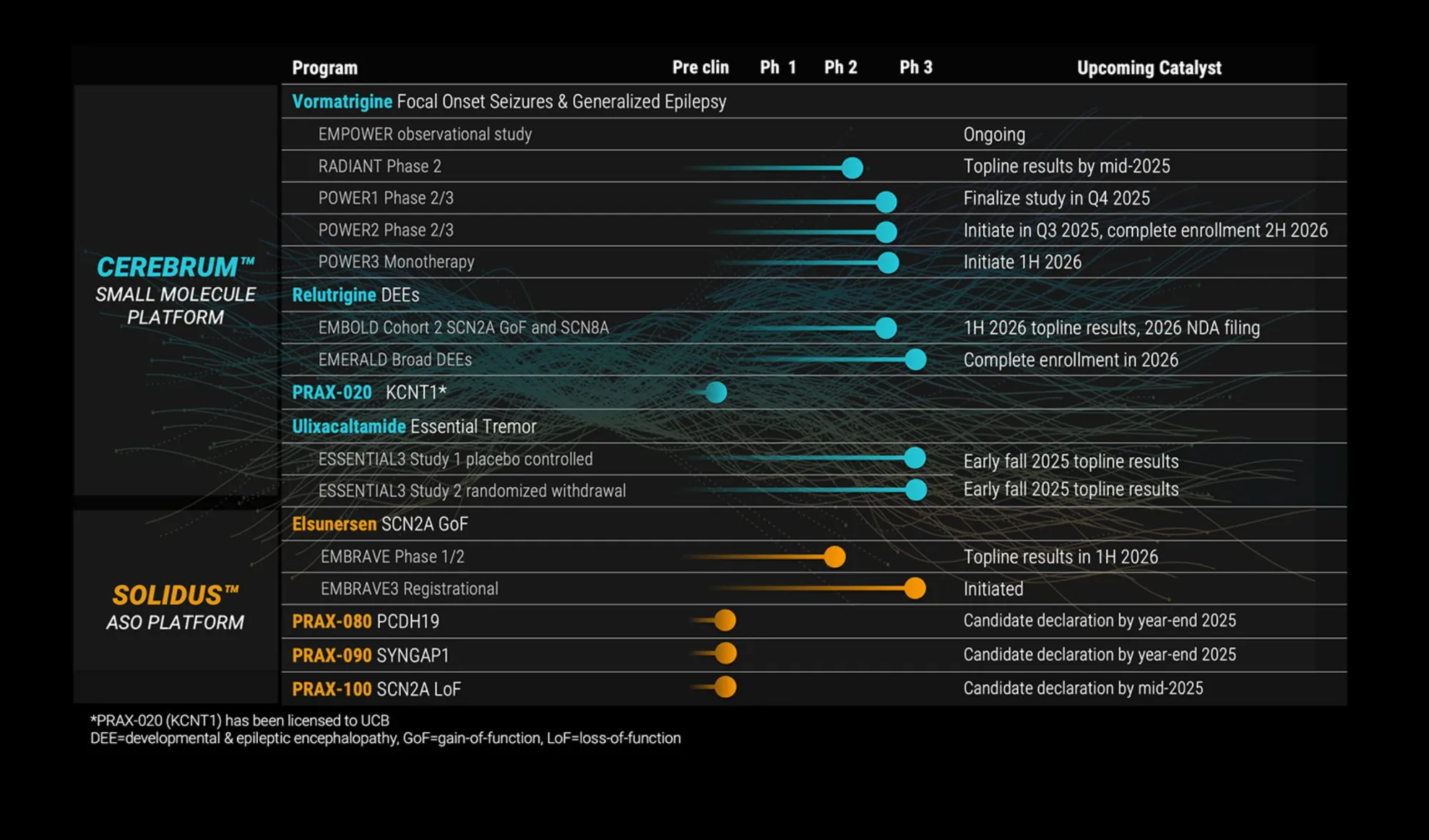
Praxis Precision Medicines announces FDA alignment for essential tremor drug and positive results for epilepsy treatment, marking major progress in neurological disorder therapies.

Praxis Precision Medicines stock rallied over 170% after its experimental essential tremor treatment ulixacaltamide exceeded expectations in two Phase 3 trials, defying earlier pessimism.