
Intellia Shares Jump After FDA Lifts Clinical Hold on Gene Therapy Trial
Intellia Therapeutics’ stock rises as the FDA clears a Phase 3 trial for its gene therapy, nexiguran ziclumeran, for a rare genetic disease.

Intellia Therapeutics’ stock rises as the FDA clears a Phase 3 trial for its gene therapy, nexiguran ziclumeran, for a rare genetic disease.

Intellia Therapeutics (NTLA) shares fell 29% premarket after the FDA paused two late-stage gene-editing trials following a patient death linked to liver complications.
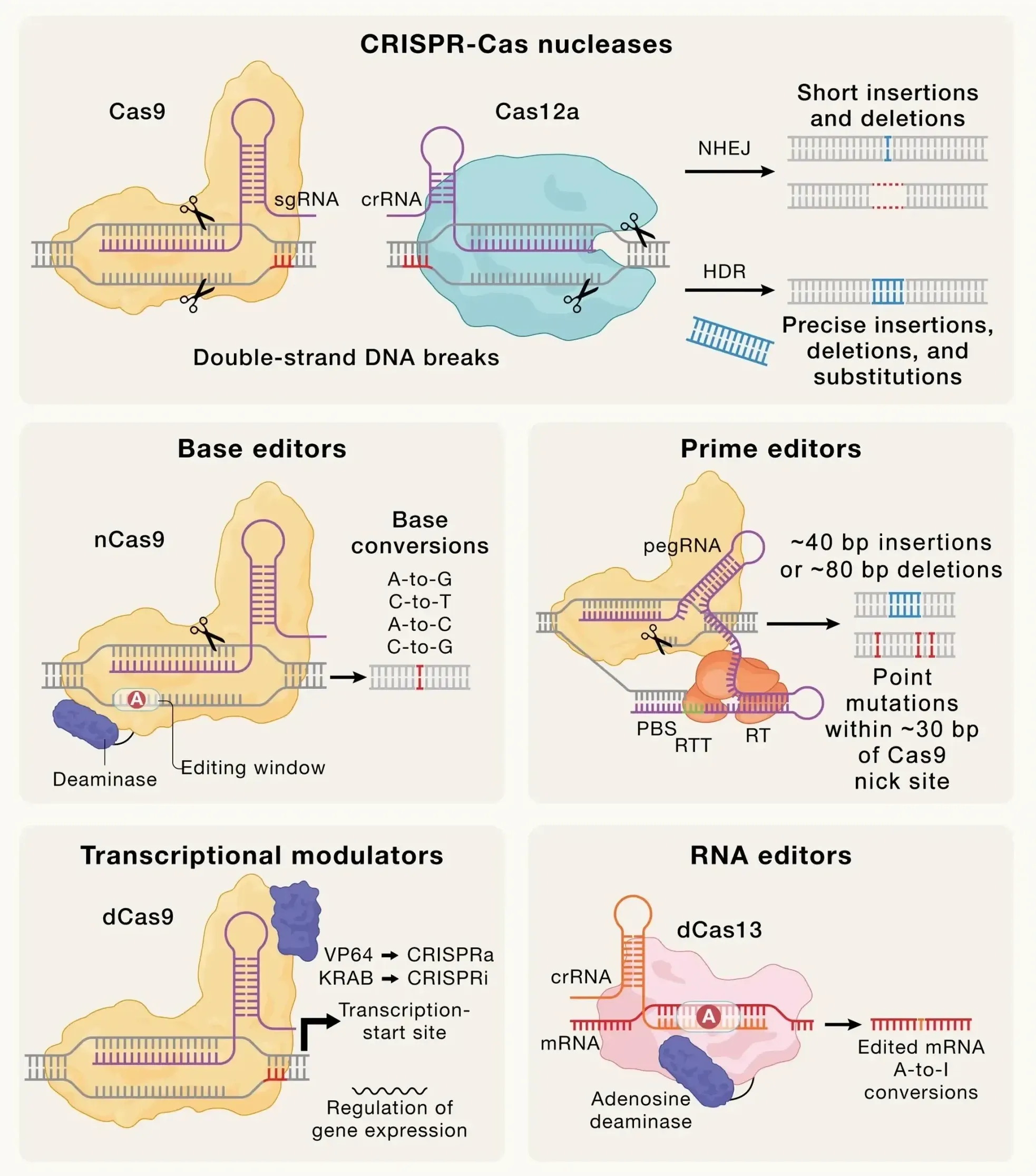
The FDA is unveiling a faster approval pathway for custom gene-editing therapies, aiming to spark major investment and accelerate cures for rare diseases through Crispr-based innovations.

Intellia Therapeutics (NTLA) shares drop 15% after FDA halts late-stage gene therapy trials following serious liver safety concerns in patient.

Intellia Therapeutics halts MAGNITUDE trials of nex-z gene therapy after patient hospitalization with Grade 4 liver toxicity. Over 650 patients enrolled in ATTR amyloidosis studies.

Intellia completed enrollment for lonvo-z, a gene-editing drug for severe swelling (HAE). It aims to be the first one-time treatment for HAE, a rare genetic disease. Top study data is expected in early 2026, with a potential U.S. launch in early 2027. Intellia's shares surged on the news.