FDA Approves New Dosing Guidelines for Afrezza Inhaled Insulin
MannKind (MNKD) receives FDA approval for updated Afrezza dosing recommendations, making it easier for patients to switch from insulin injections to inhaled insulin therapy.

MannKind (MNKD) receives FDA approval for updated Afrezza dosing recommendations, making it easier for patients to switch from insulin injections to inhaled insulin therapy.
Wells Fargo upgrades Merck to Overweight; Truist and H.C. Wainwright initiate coverage on BeOne Medicines, MeiraGTx, MannKind, and Fulcrum with Buy ratings and promising price targets.
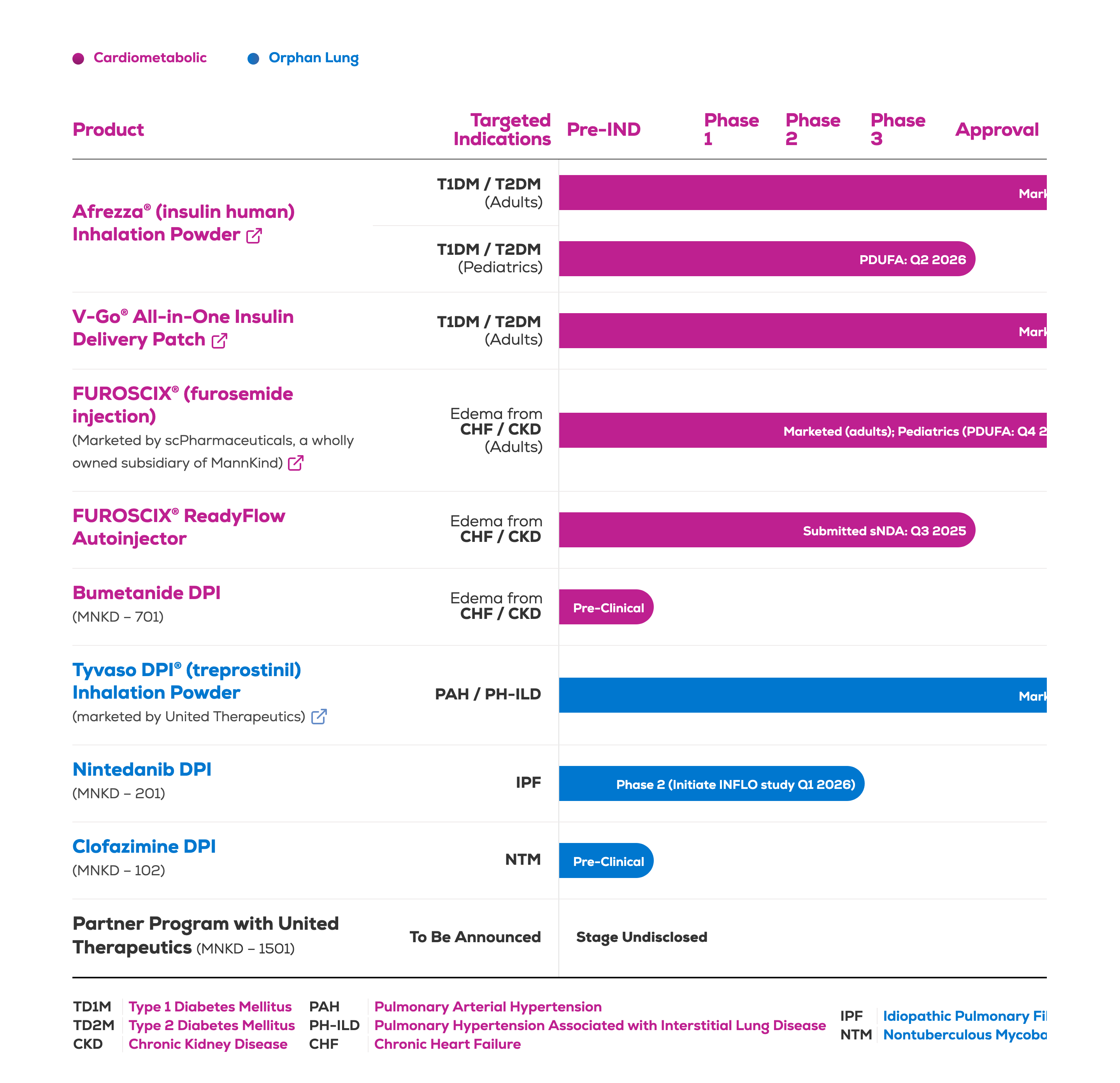
MannKind Corporation discontinues its Phase 3 ICoN-1 trial for nebulized clofazimine (MNKD-101) in NTM lung disease after futility analysis shows no efficacy. The company will focus on developing its dry powder version, MNKD-102.
United Therapeutics' Tyvaso Inhalation Solution demonstrates significant benefits in the TETON-2 study for Idiopathic Pulmonary Fibrosis (IPF), offering new hope and potential treatment advancements for this debilitating lung condition.
MannKind Corporation acquires scPharmaceuticals, significantly expanding its presence in cardiometabolic medicine with FUROSCIX, an FDA-approved treatment for heart and kidney disease. This strategic move aims to diversify revenue and accelerate growth.