
MoonLake's Sonelokimab Gets FDA Fast Track for Rare Skin Disease
MoonLake Immunotherapeutics receives FDA Fast Track designation for sonelokimab in palmoplantar pustulosis, accelerating development for this untreated skin condition.

MoonLake Immunotherapeutics receives FDA Fast Track designation for sonelokimab in palmoplantar pustulosis, accelerating development for this untreated skin condition.
Goldman Sachs downgraded MoonLake Immunotherapeutics to Sell, citing high approval risks despite progress in Phase 3 trials.
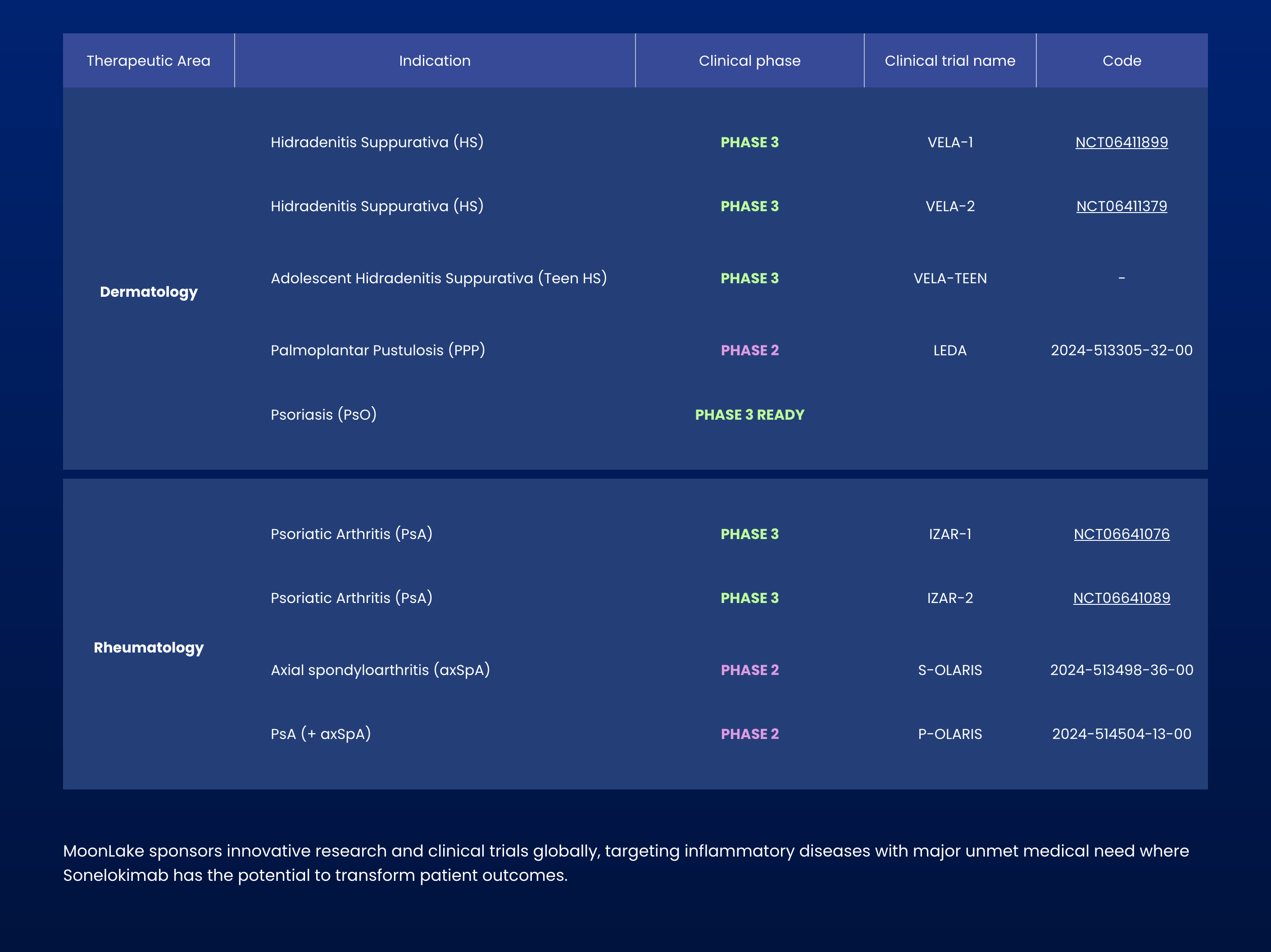
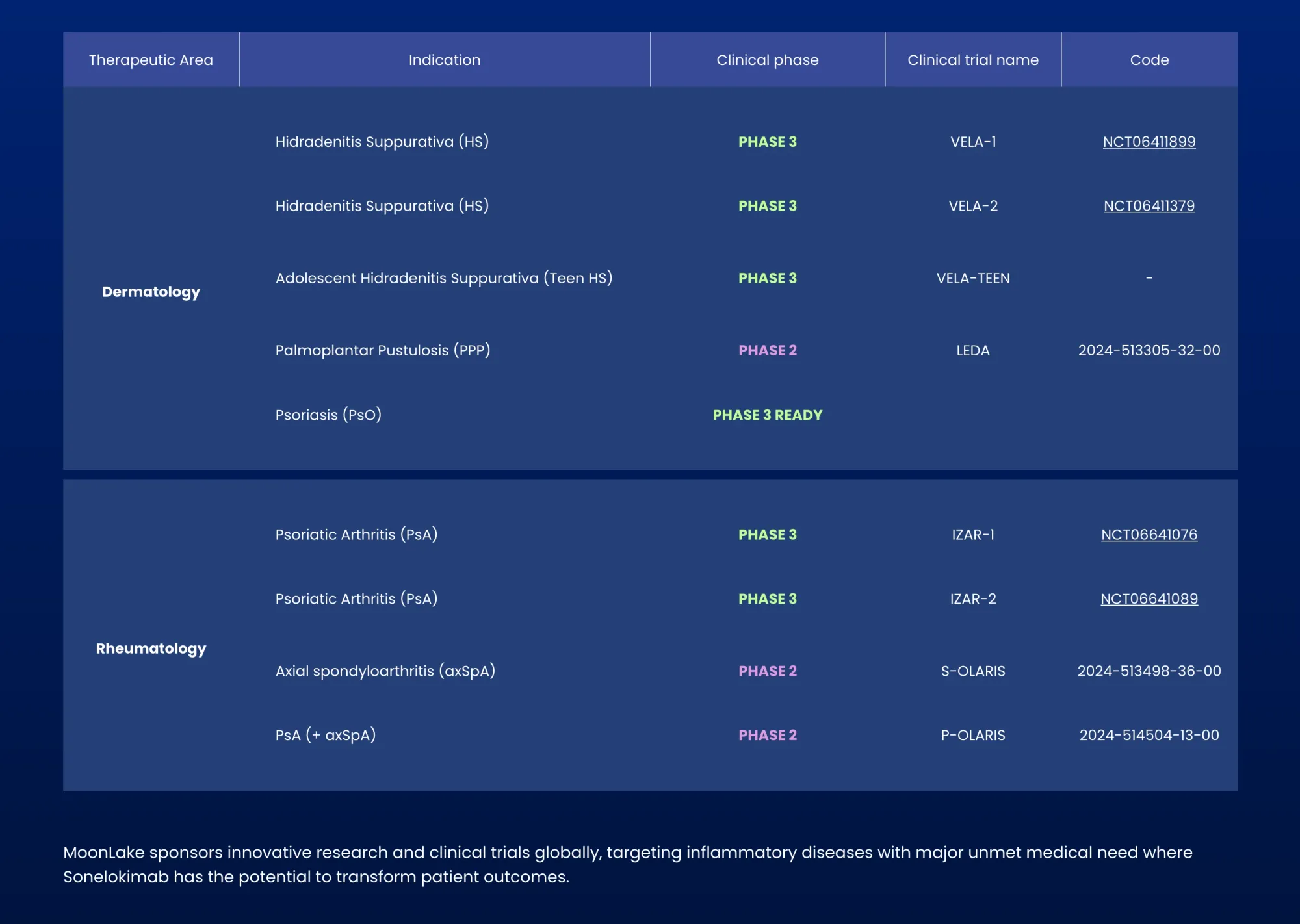
MoonLake Immunotherapeutics receives positive FDA feedback, clearing pathway to submit drug application for hidradenitis suppurativa treatment without additional trials needed.
FDA plans to require only one pivotal trial for drug approvals instead of two, potentially accelerating development timelines while raising safety and efficacy concerns.
Oruka Therapeutics shares jump 30% as analysts predict gains following competitor MoonLake's Phase 3 results.
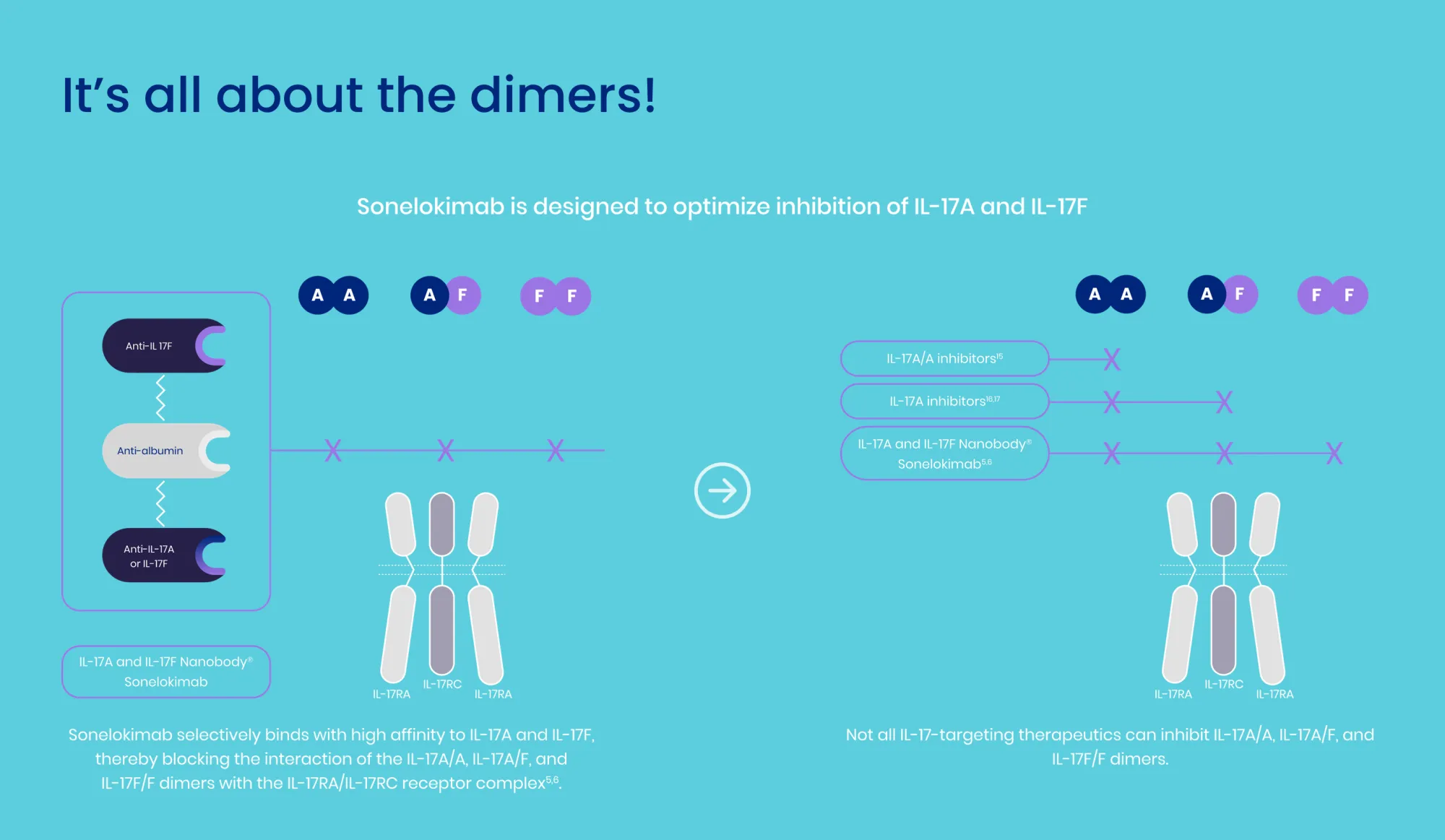
MoonLake announced Phase 3 results for sonelokimab (VELA trials) in HS. While one trial succeeded using the primary analysis, the other faced challenges due to a high placebo response, leading to a stock downgrade.