FDA Rejects Disc Medicine's Drug for Rare Sunlight Sensitivity Disorder, Shares Plunge
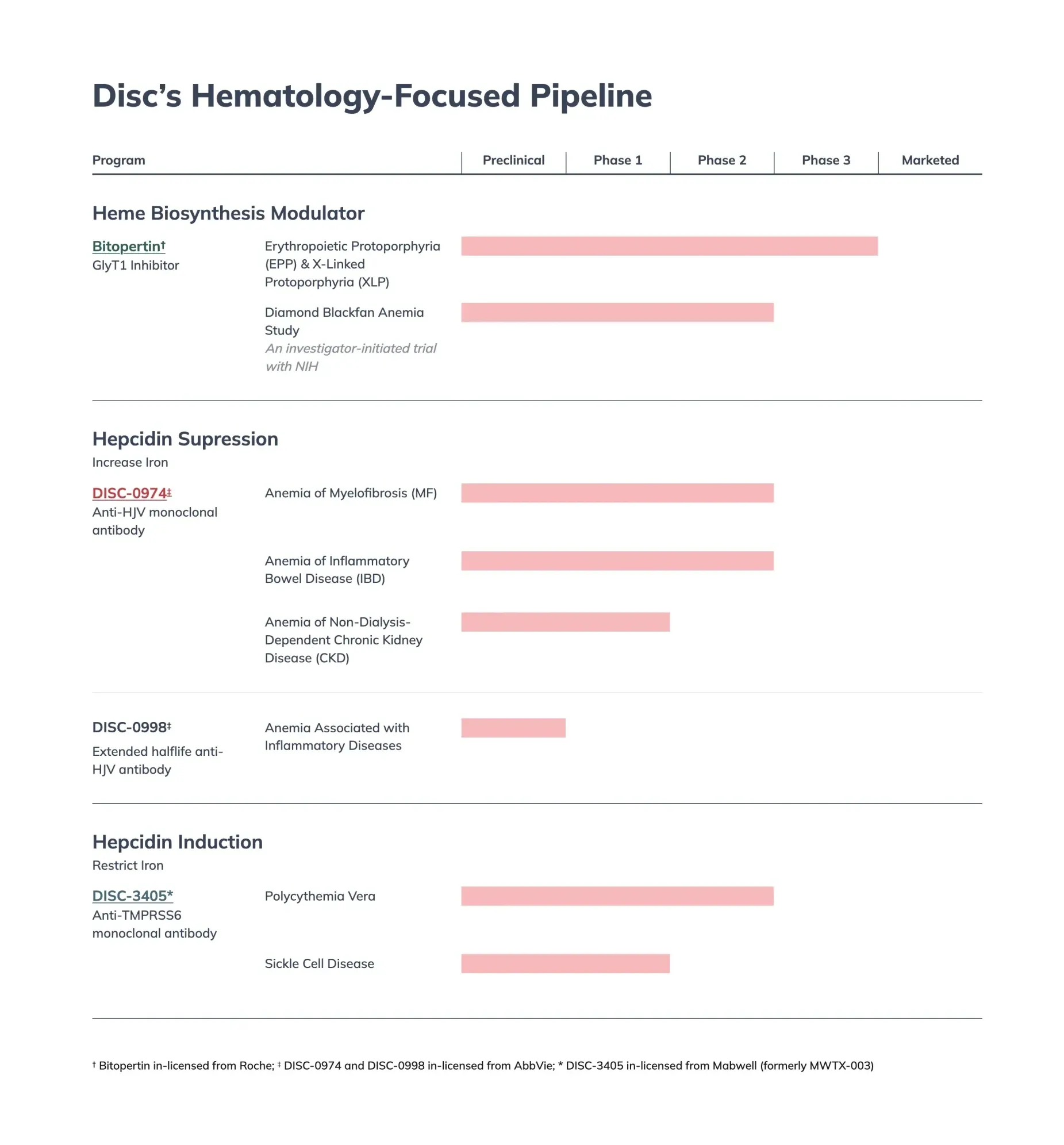
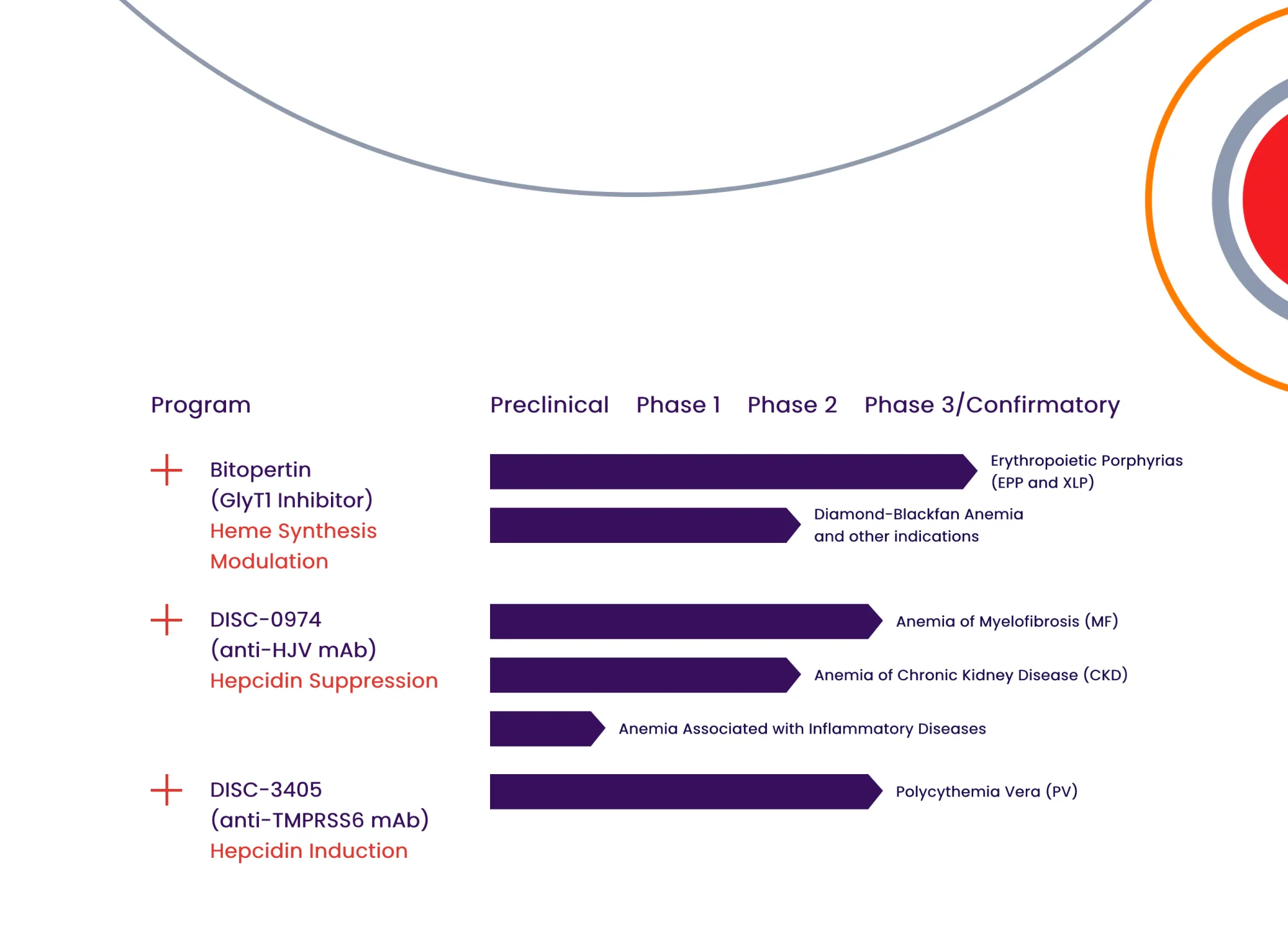
Disc Medicine's bitopertin gets turned down by FDA for treating EPP, a painful genetic condition making skin ultra-sensitive to sun.

Disc Medicine's bitopertin gets turned down by FDA for treating EPP, a painful genetic condition making skin ultra-sensitive to sun.

FDA extends review timelines for Eli Lilly's obesity pill Orforglipron and three other priority voucher drugs, despite promises of accelerated 1-2 month reviews under new program.
Disc Medicine (IRON) shares fall 14% after FDA official questions bitopertin's effectiveness for rare blood disorder, though analysts maintain buy ratings and approval pathway optimism.
Disc Medicine receives FDA's Commissioner's National Priority Voucher for bitopertin, accelerating approval timeline to 1-2 months for EPP patients who suffer severe sun sensitivity.