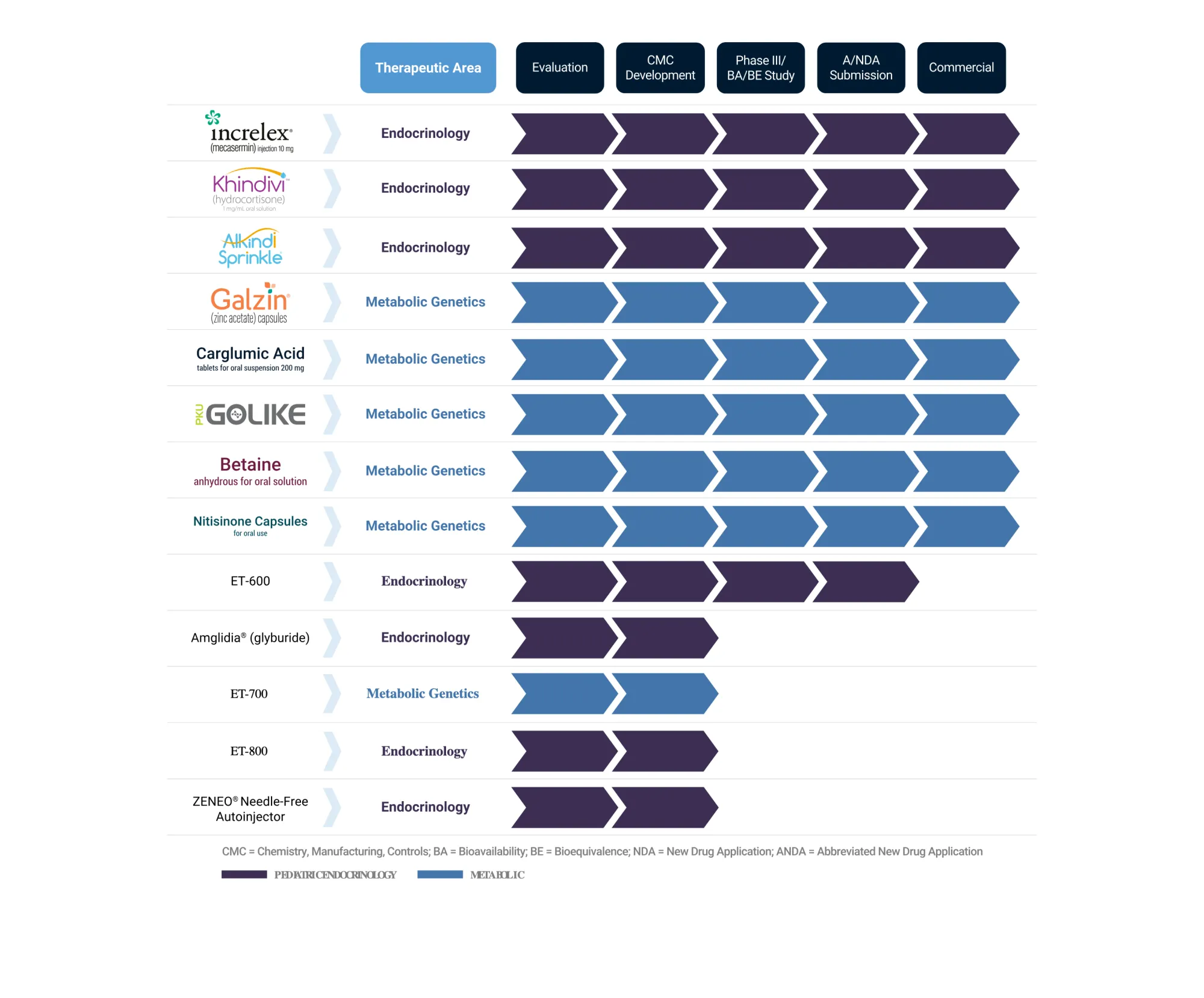
Eton Pharmaceuticals Secures Rights to First Generic Treatment for Ultra-Rare Disease
Eton Pharmaceuticals licenses U.S. rights to an ultra-rare disease treatment affecting under 100 patients, expecting FDA approval and mid-2026 launch as first generic alternative.
