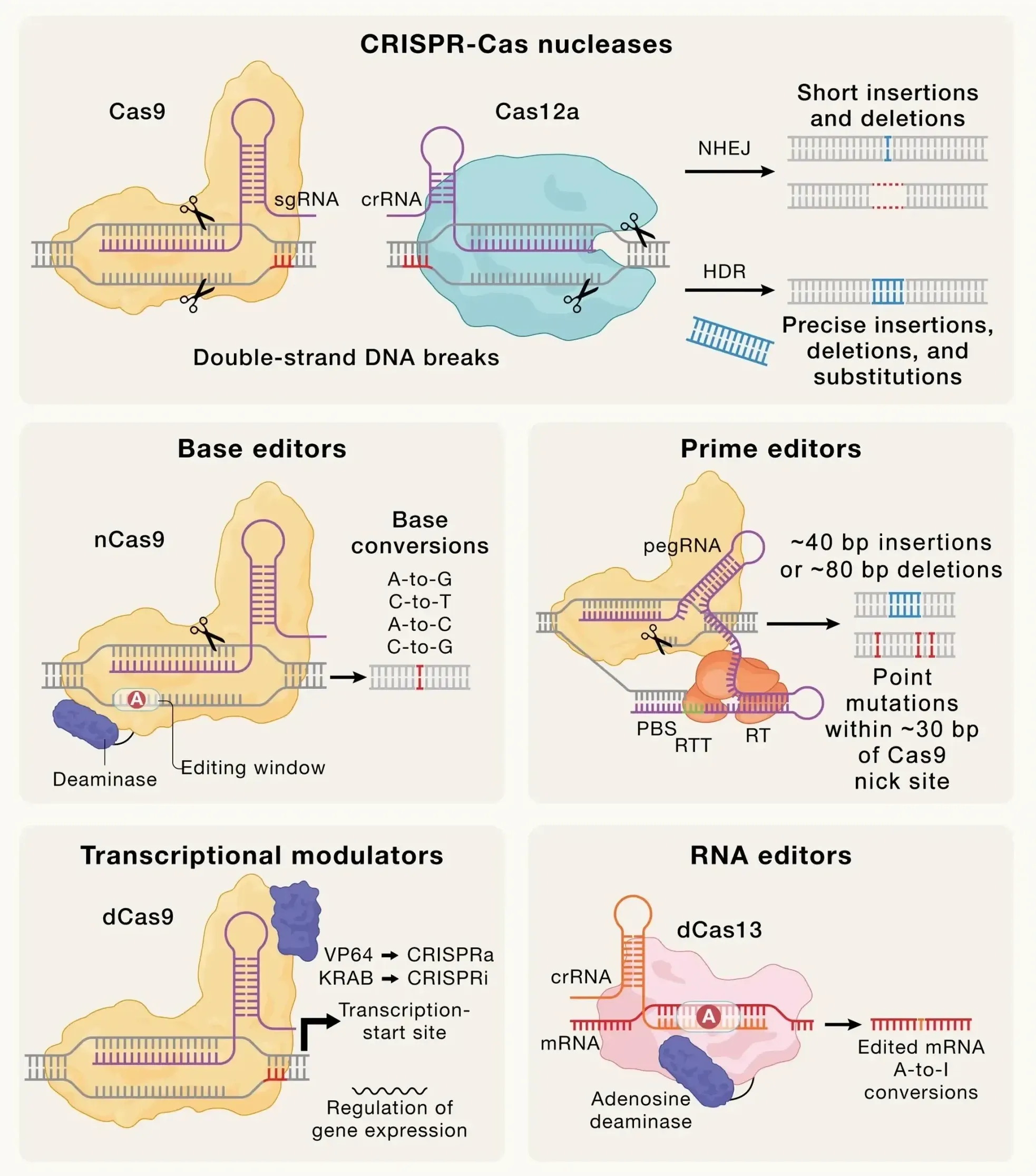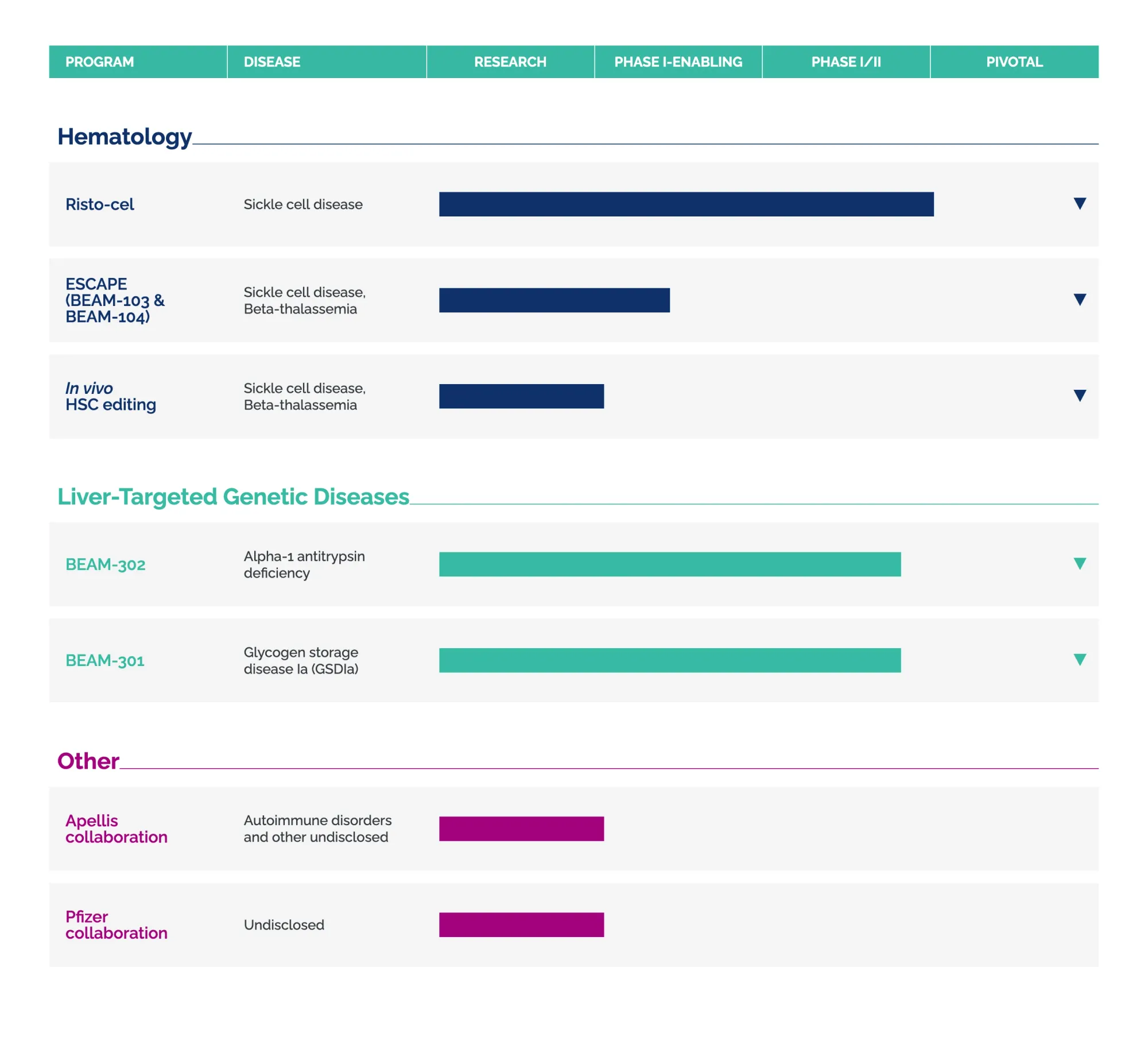
William Blair Bullish on Beam Therapeutics Following Strategic Update
William Blair maintains Outperform rating on Beam Therapeutics after company announces FDA alignment for BEAM-302 and planned risto-cel application, citing clear regulatory pathway ahead.