
Amgen Beats Q4 Expectations Despite FDA Drug Controversy
Amgen (AMGN) reports strong Q4 2025 earnings with $9.87B revenue, beating estimates. Company defies FDA request to withdraw Tavneos while advancing obesity drug MariTide through trials.

Amgen (AMGN) reports strong Q4 2025 earnings with $9.87B revenue, beating estimates. Company defies FDA request to withdraw Tavneos while advancing obesity drug MariTide through trials.

Zenas BioPharma stock plunges over 50% after Phase 3 obexelimab trial results disappoint Wall Street, despite meeting all endpoints in rare autoimmune disease study.

Novo Nordisk's Wegovy pill becomes the first FDA-approved oral GLP-1 weight-loss medication, launching January 2026 at $149/month as the Danish drugmaker aims to reclaim market share from Eli Lilly.

Amneal Pharmaceuticals and mAbxience announce FDA approval of Boncresa and Oziltus, biosimilar alternatives to Prolia and XGEVA for bone health treatment.

Nine pharmaceutical giants including Merck and GSK agree to slash U.S. drug prices to match other wealthy nations under new Trump administration deals, offering discounts up to 70%.
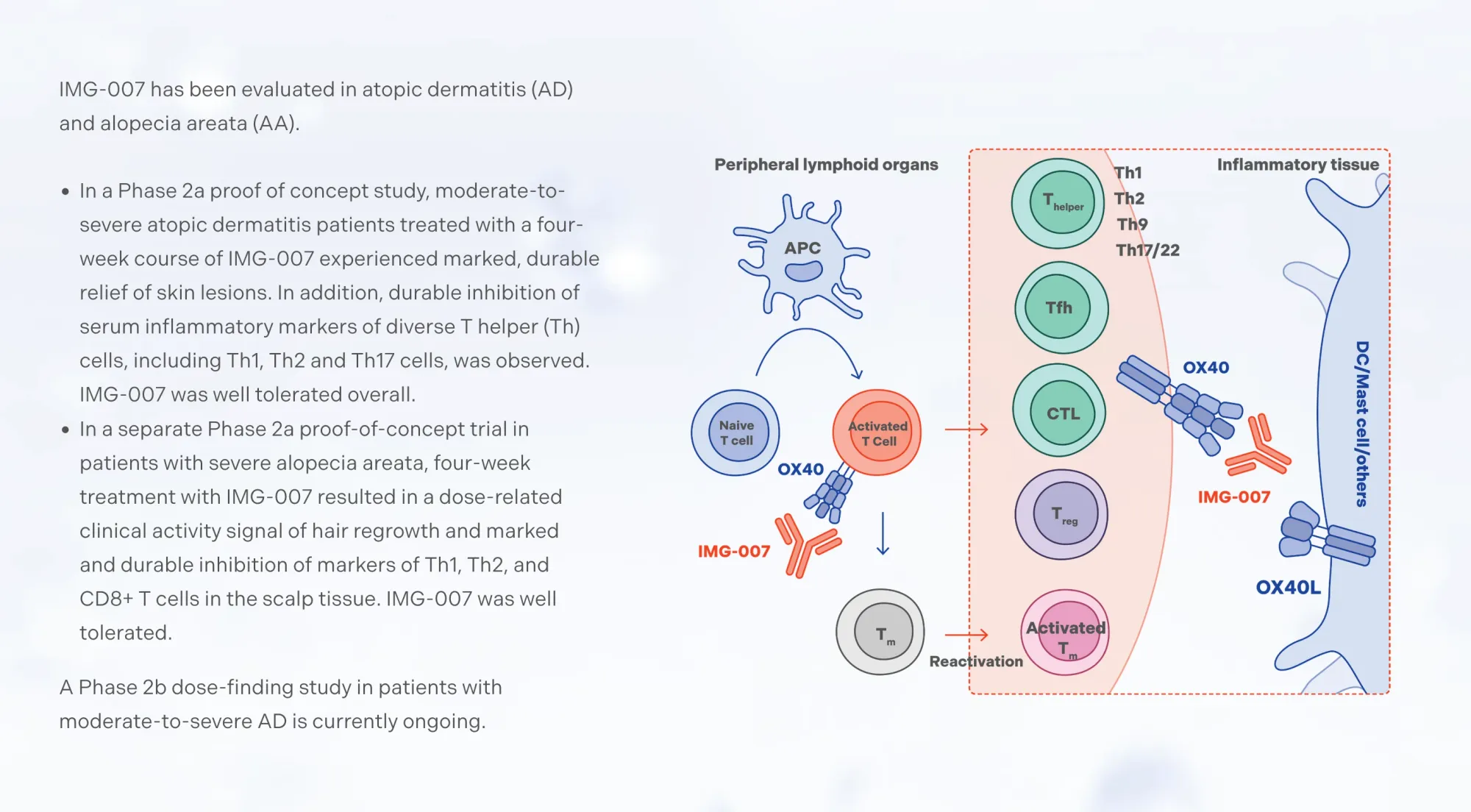
Leerink rates ImageneBio stock Outperform with $30 target, calling it "undervalued."