FDA Approves AQVESME: First Treatment for Thalassemia Anemia
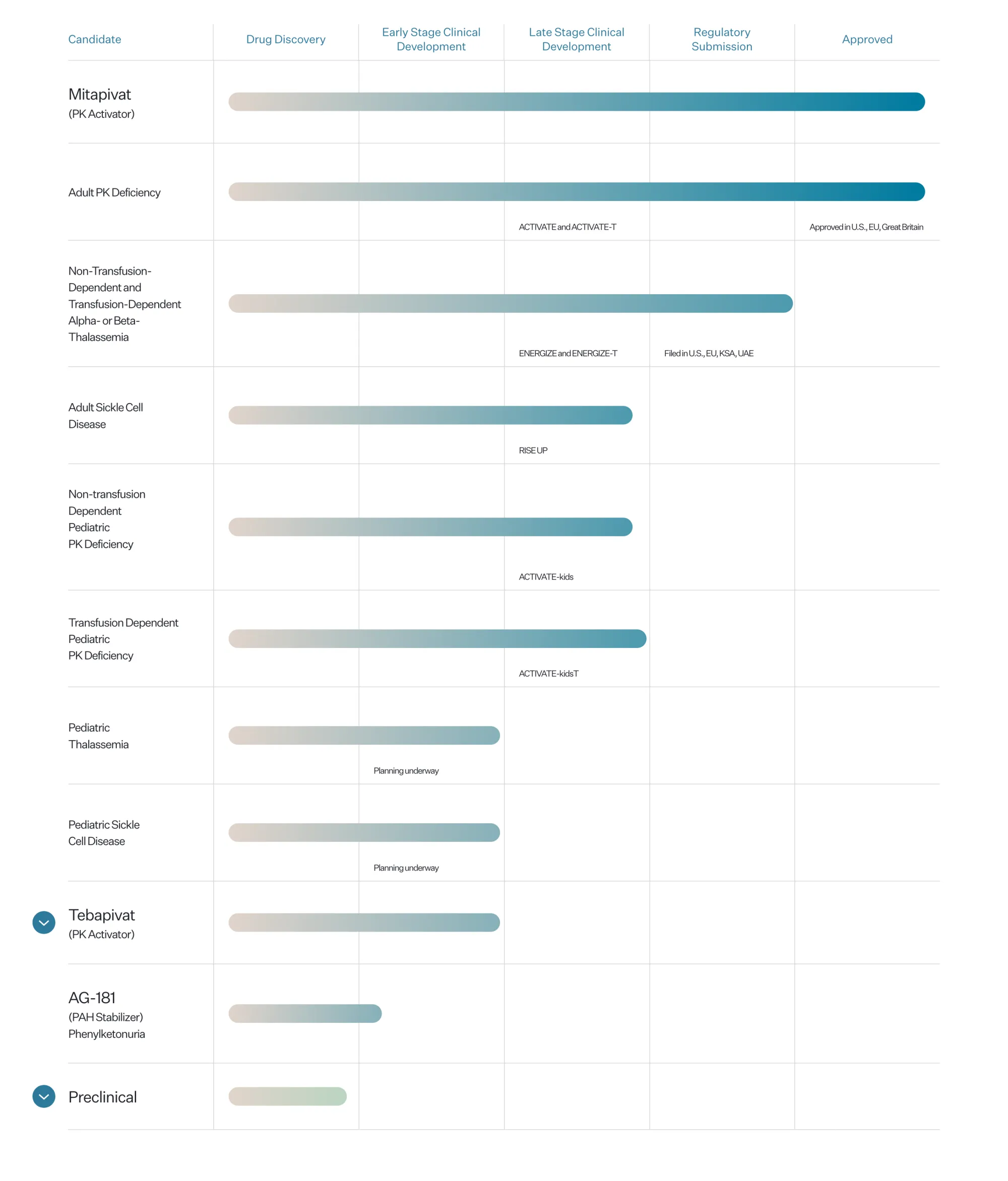
The FDA has approved Agios’ AQVESME (mitapivat), the first medicine for treating anemia in adults with alpha or beta-thalassemia, offering hope for thousands of patients.

The FDA has approved Agios’ AQVESME (mitapivat), the first medicine for treating anemia in adults with alpha or beta-thalassemia, offering hope for thousands of patients.

Fulcrum Therapeutics rises as Agios’ RISE UP trial falls short. Analysts say results reduce competition in sickle cell disease and strengthen Fulcrum’s position ahead of new PIONEER data.

Agios Pharmaceuticals reports mixed Phase 3 results for mitapivat in sickle cell disease, meeting some endpoints while missing key pain crisis reduction goals.
The FDA extended PYRUKYND's review for thalassemia by 3 months to Dec 7, 2025, after Agios submitted a plan (REMS) to manage liver injury risk. This oral medicine aims to treat adult alpha- or beta-thalassemia.