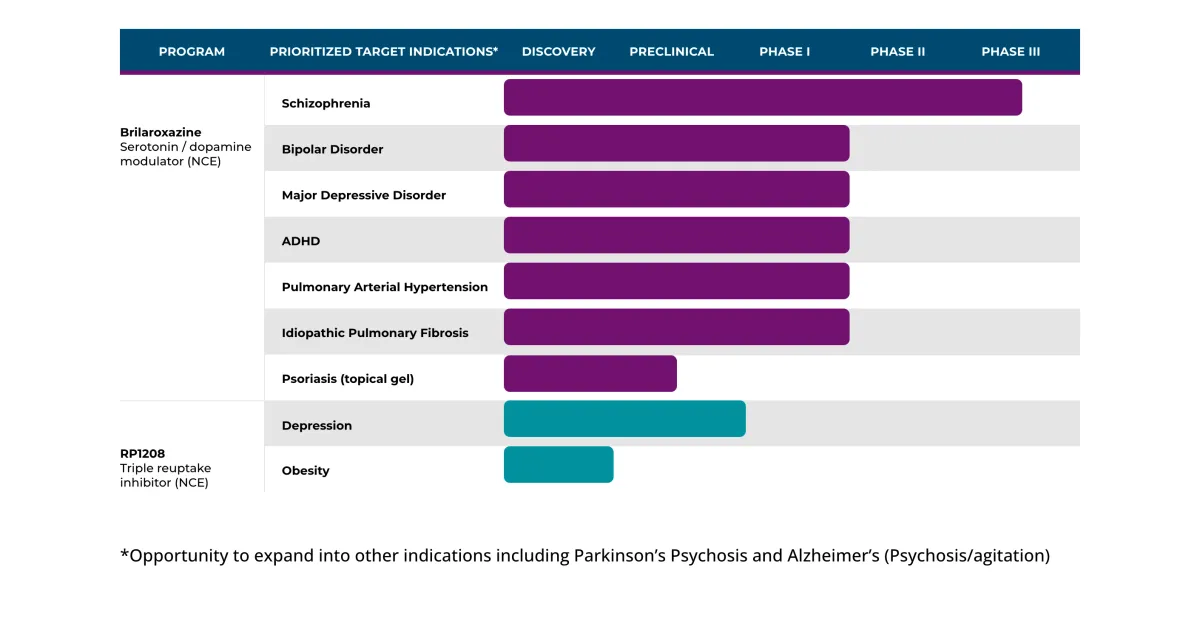
Reviva's Schizophrenia Drug Faces Major Setback: FDA Demands Second Phase 3 Trial
FDA rejects Reviva's NDA plans for brilaroxazine, requiring additional Phase 3 trial. Launch delayed until at least 2026+, contingent on securing funding for the study.
Already have an account? Sign in.
