PTC SEPHIENCE: A New PKU Treatment Approved
FDA-approved SEPHIENCE (sepiapterin), an oral drug, treats PKU in patients 1 month+. SEPHIENCE aims to be the future standard of care.

FDA-approved SEPHIENCE (sepiapterin), an oral drug, treats PKU in patients 1 month+. SEPHIENCE aims to be the future standard of care.

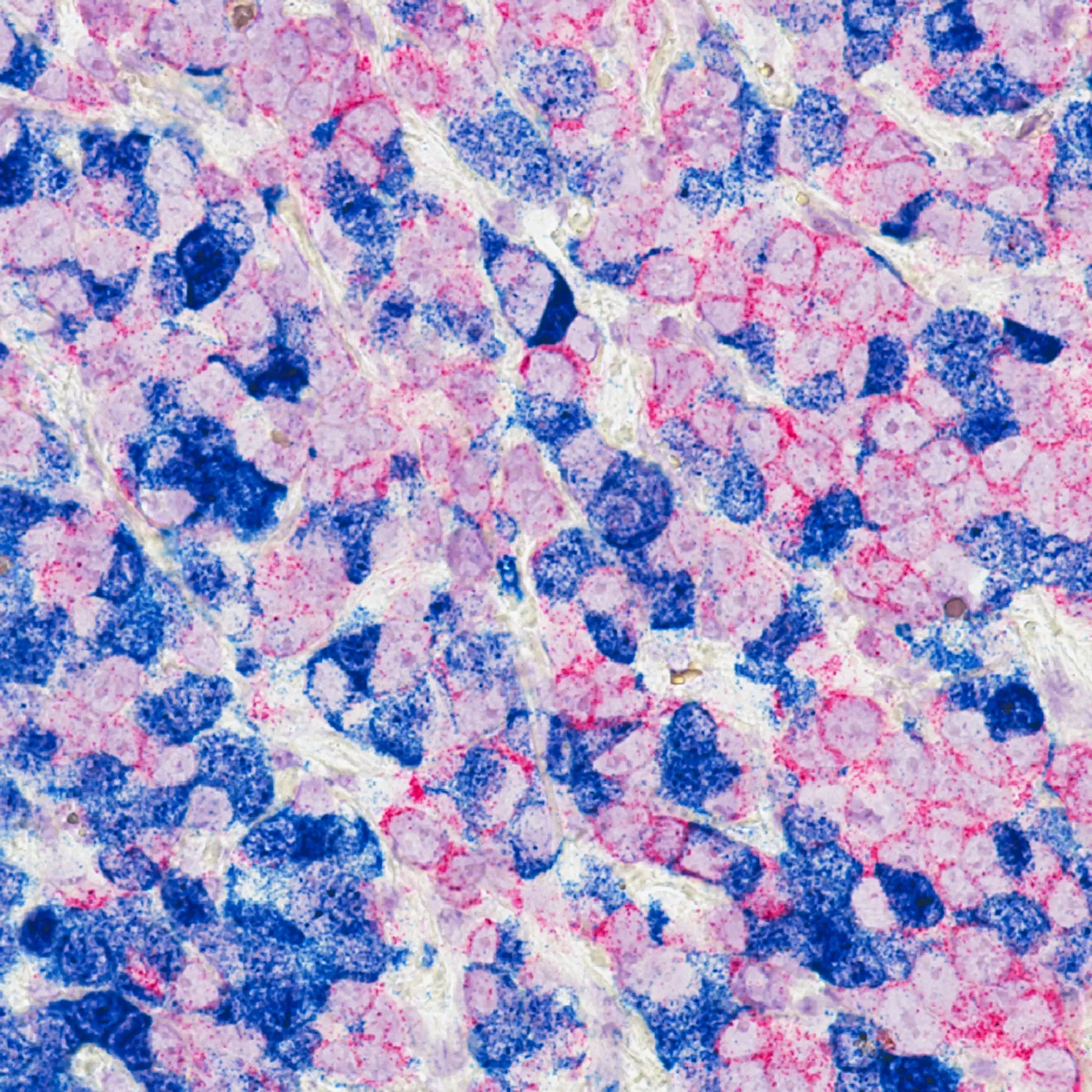
BioNTech's new "smart bomb" cancer drug, trastuzumab pamirtecan, excelled in trials for HER2+ breast cancer, beating an older therapy. This marks BioNTech's first major oncology success and offers a promising new option for patients, globally.
CERo's AML drug, CER-1236, received FDA Fast Track & Orphan Drug status and is in a Phase 1/1b trial. CERo Therapeutics faces a Nasdaq delisting challenge due to low stockholders' equity, but has appealed and plans to raise funds.
Cretostimogene, a new bladder cancer treatment, shows durable 24-month complete responses (41.8%) in high-risk patients unresponsive to BCG. It has a good safety profile and offers bladder-sparing potential, with approval submission planned.