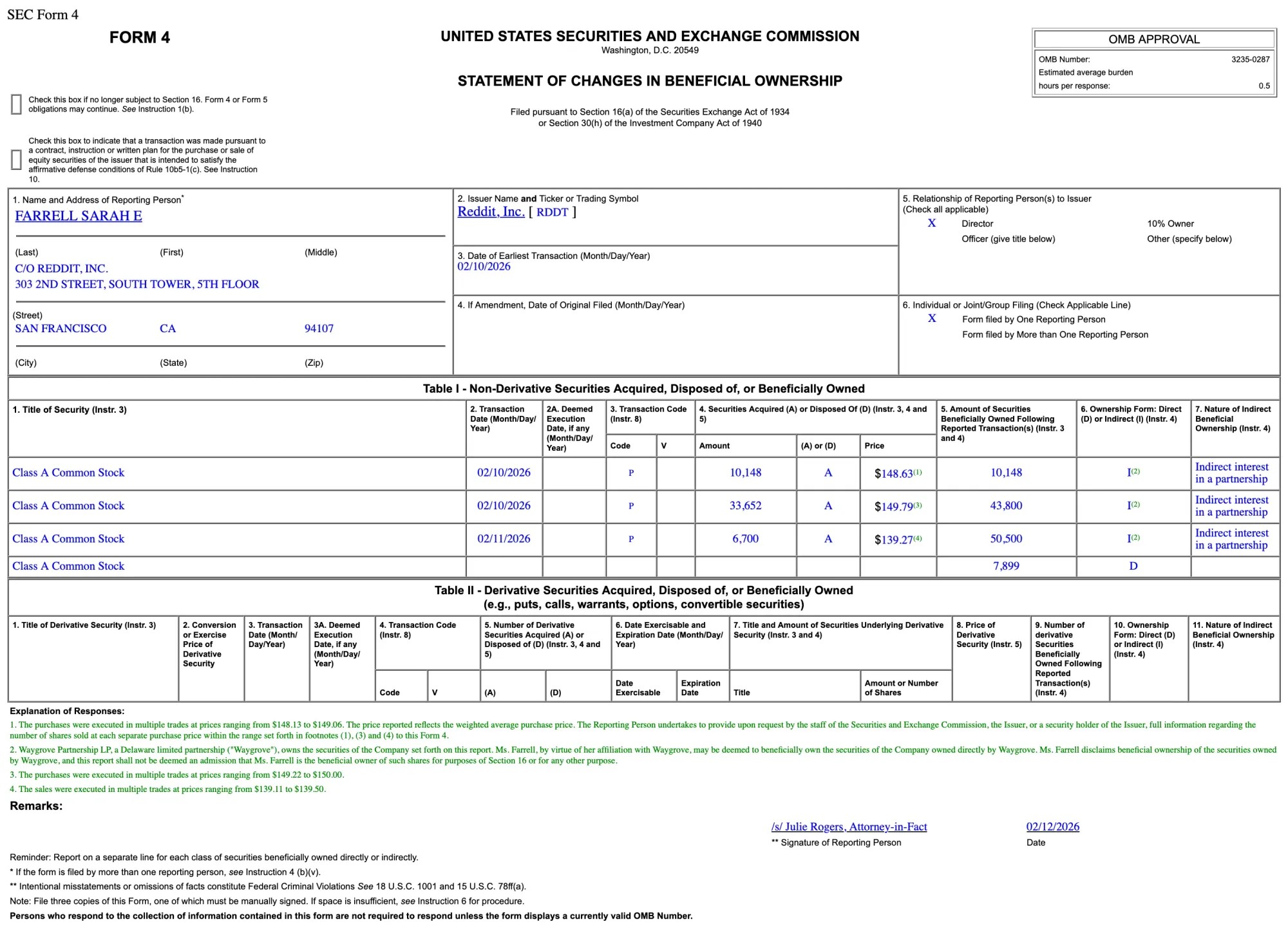
FDA Approves Amneal’s New Epinephrine Injection Vials for Emergency Care
The FDA has approved Amneal Pharmaceuticals’ new single- and multi-dose epinephrine vials, expanding access to a critical emergency medicine used for allergic reactions and septic shock in hospitals and acute care settings.