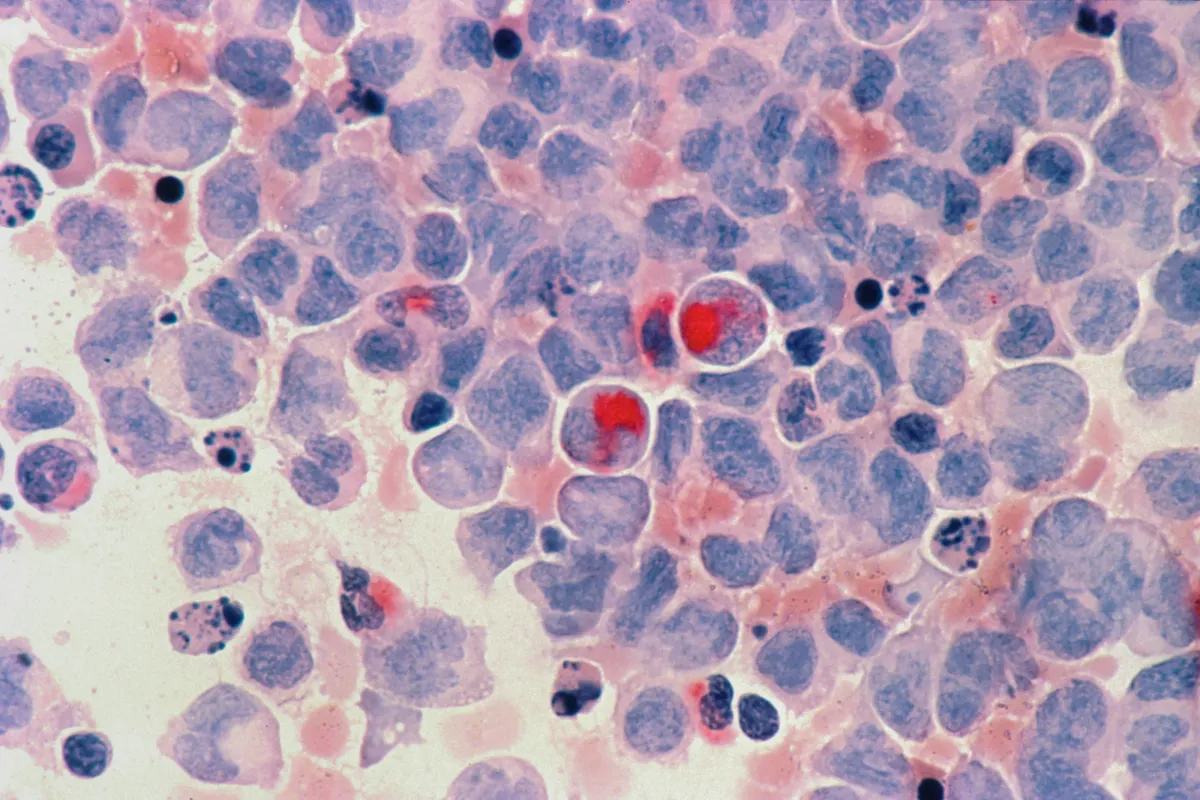
FDA Approves Revuforj for Relapsed NPM1 Mutated Acute Myeloid Leukemia
FDA has approved Revuforj (revumenib) for use in adult and pediatric patients with relapsed or refractory NPM1-mutated acute myeloid leukemia. However, the agency has warned of serious and potentially fatal side effects, including differentiation syndrome.
Already have an account? Sign in.



