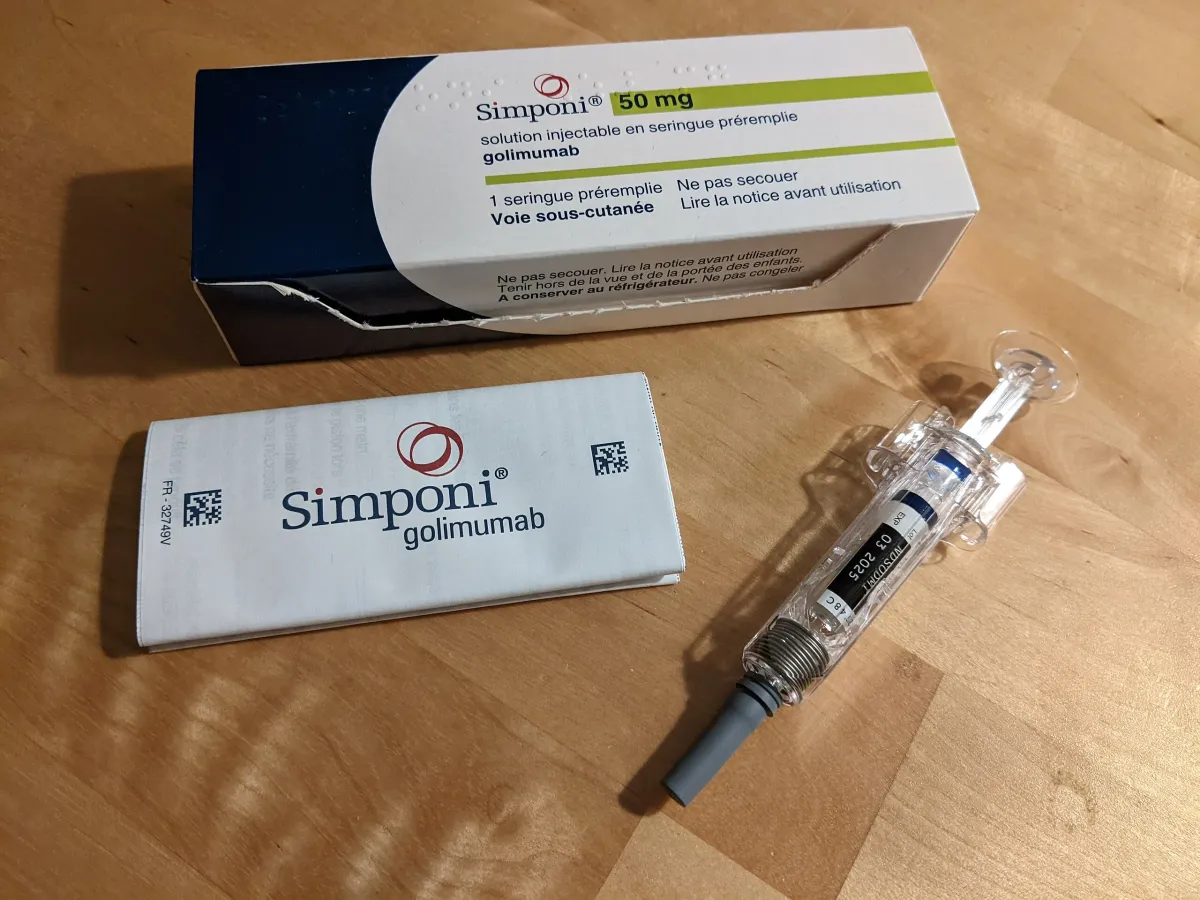
Alvotech's Biosimilar Drug Faces FDA Setback Due to Manufacturing Issues
Alvotech receives FDA complete response letter for AVT05 biosimilar to Simponi, citing manufacturing facility deficiencies. Company revises 2025 revenue outlook downward.
Already have an account? Sign in.
